Abstract
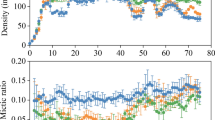
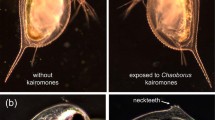
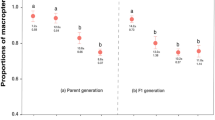
This study examines two aspects of predator-induced, morphological defense in rotifers: developmental time lags for acquisition and loss of the defense, and cost of the defense. When the predator and its inducing kairomone disappear from a community, the extent to which prey population growth is inhibited by the retention of the induced defense will be determined by the reversibility of the defense and the magnitude of any cost associated with the defense. In Brachionus calyciflorus, Asplanchna-induced posterior spines were lost more slowly than acquired. When females of the induced morph were removed from Asplanchna kairomone after oviposition of their first egg, they continued to produce long-spined offspring throughout their life. Even when cultured from birth to death without Asplanchna kairomone, females born with long, induced spines produced daughters with some induced spine development. In contrast, when females of the basic (non-induced) morph were exposed to Asplanchna kairomone after oviposition of their first egg, some of their second and all of their subsequently oviposited eggs developed into daughters with long spines. In B. calyciflorus and also Keratella tropica, the reproductive cost of Asplanchna-induced defense was determined by comparing population growth rates of basic and Asplanchna-induced morphs. Populations were initiated with individuals of either morph, and Asplanchna was present with the induced morph to assure that all individuals born during the culture period had long spines. In each of three separate experiments with each rotifer species, population growth rates of basic and induced morphs were statistically indistinguishable. Thus, possession of long spines in the absence of Asplanchna is unlikely to inhibit the reproduction of these rotifers through any allocation or energetic cost.





Similar content being viewed by others
References
Aránguiz-Acuña A, Ramos-Jiliberto R, Sarma N, Sarma SSS, Bustamente R, Toledo V (2010) Benefits, costs and reactivity of inducible defences: an experimental test with rotifers. Freshw Biol 55:2114–2122
Diéguez MC, Gilbert JJ (2011) Daphnia-rotifer interactions in Patagonian communities. Hydrobiologia 662:189–195
Gabriel W (1999) Evolution of reversible plastic responses: inducible defenses and environmental tolerance. In: Tollrian R, Harvell CD (eds) The ecology and evolution of inducible defenses. Princeton University Press, Princeton, NJ, pp 286–305
Gilbert JJ (1966) Rotifer ecology and embryological induction. Science 151:1234–1237
Gilbert JJ (1967) Asplanchna and posterolateral spine induction in Brachionus calyciflorus. Arch Hydrobiol 64:1–62
Gilbert JJ (1980) Further observations on developmental polymorphism and its evolution in the rotifer Brachionus calyciflorus. Freshw Biol 10:281–294
Gilbert JJ (1999) Kairomone-induced morphological defenses in rotifers. In: Tollrian R, Harvell CD (eds) The ecology and evolution of inducible defenses. Princeton University Press, Princeton, NJ, pp 127–141
Gilbert JJ (2009) Predator-specific inducible defenses in the rotifer Keratella tropica. Freshw Biol 54:1933–1946
Gilbert JJ (2011) Induction of different defences by two enemies in the rotifer Keratella tropica: response priority and sensitivity to enemy density. Freshw Biol 56:926–938
Gilbert JJ, Schröder T (2004) Rotifers from diapausing, fertilized eggs: unique features and emergence. Limnol Oceanogr 49:1341–1354
Halbach U (1970) Die Ursachen der Temporalvariation von Brachionus calyciflorus Pallas (Rotatoria). Oecologia 4:262–318
Marinone MC, Zagarese HE (1991) A field and laboratory study on factors affecting polymorphism in the rotifer Keratella tropica. Oecologia 86:372–377
Pavón-Meza EL, Sarma SSS, Nandini S (2007) Combined effects of temperature, food availability and predator’s (Asplanchna girodi) allelochemicals on the demography and population growth of Brachionus havanaensis (Rotifera). Allelopath J 21:95–106
Pohnert G, Steinke M, Tollrian R (2007) Chemical cues, defence metabolites and the shaping of pelagic interspecific interactions. Trends Ecol Evol 22:198–204
Sarma SSS, Resendiz RAL, Nandini S (2011) Morphometric and demographic responses of brachionid prey (Brachionus calyciflorus Pallas and Plationus macracanthus (Daday)) in the presence of different densities of the predator Asplanchna brightwelli (Rotifera: Asplanchnidae). Hydrobiologia 662:179–187
Schröder T, Gilbert JJ (2009) Maternal age and spine development in the rotifer Brachionus calyciflorus: increase of spine length with birth order. Freshw Biol 54:1054–1065
Stemberger RS (1981) A general approach to the culture of planktonic rotifers. Can J Fish Aquat Sci 38:721–724
Stemberger RS (1988) Reproductive costs and hydrodynamic benefits of chemically induced defenses in Keratella testudo. Limnol Oceanogr 33:593–606
Stemberger RS (1990) Food limitation, spination, and reproduction in Brachionus calyciflorus. Limnol Oceanogr 35:33–44
Tollrian R, Dodson SI (1999) Inducible defenses in Cladocera: constraints, costs, and multipredator environments. In: Tollrian R, Harvell CD (eds) The ecology and evolution of inducible defenses. Princeton University Press, Princeton, NJ, pp 177–202
Tollrian R, Harvell CD (eds) (1999a) The ecology and evolution of inducible defenses. Princeton University Press, Princeton, NJ
Tollrian R, Harvell CD (1999b) The evolution of inducible defenses: current ideas. In: Tollrian R, Harvell CD (eds) The ecology and evolution of inducible defenses. Princeton University Press, Princeton, NJ, pp 306–321
Van Donk E (2007) Chemical information transfer in freshwater plankton. Ecol Info 2:112–120
Zagarese HE, Marinone MC (1992) Induction and inhibition of spine development in the rotifer Keratella tropica: evidence from field observations and laboratory experiments. Freshw Biol 28:289–300
Acknowledgments
I am most grateful to Piet Spaak and an anonymous referee for many important and helpful comments, and to Dartmouth College and its Department of Biological Sciences for research support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Piet Spaak.
Rights and permissions
About this article
Cite this article
Gilbert, J.J. Predator-induced defense in rotifers: developmental lags for morph transformations, and effect on population growth. Aquat Ecol 46, 475–486 (2012). https://doi.org/10.1007/s10452-012-9416-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-012-9416-x