Abstract
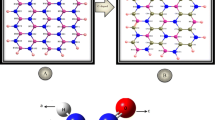
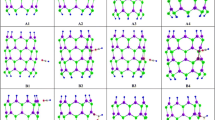
A density function theory (DFT) is applied to investigate the interaction and adsorption of nitrate ion on the exterior and interior surface of the pristine, Al and Ga-doped BNNTs. The calculated results indicate that the values of adsorption energy and enthalpy of the NO3−@ Al-doped BNNTs complex is more negative than pristine and Ga-doped. The adsorption energy nitrate ion on the surface of BNNTs is in order Al-doped > Ga-doped > pristine. This result demonstrates that the adsorption of nitrate ion on the surface of Al-doped BNNTs is stronger than Ga doped and pristine states. The chemical potential (µ) values for nitrate ion adsorption on the pristine, Al and Ga doped BNNTs are negative and is in order µpristine > µAl-doped > µGa-doped, it means that these compounds are stable. The values of ▽2ρ(BCP) and H(BCP) for [(NO3)O…B(BNNTs)] at the all adsorption models are positive and the |V/G| ratio for all models is > 2, it denotes the strong electrostatic interaction between nitrate ion with nanotube. In addition, the results of natural bonding orbital (NBO) and maximum charge transfer parameters (∆N) indicate that at all adsorption models, the charge transfer occurs from nitrate ion toward nanotube and nanotube acts as p-type semiconductor.






Similar content being viewed by others
References
Ahmadi Peyghan, A., Baei, M.T., Moghimi, M., Hashemian, S.: Adsorption and electronic structure study of imidazole on (6,0) zigzag single-walled boron nitride nanotube. J. Clust. Sci. 24, 31–47 (2013)
Ahmadi Peyghan, A., Aslanzadeh, S.A., Samiei, A.: Ammonia borane reaction with a BN nanotube: a hydrogen storage route. Monatsh. Chem. 145, 1083–1087 (2014)
Bader, R.F.W.: Atoms in Molecules: A Quantum Theory. Oxford University Press, Oxford (1990)
Bulat, F.A., Toro-Labbé, A., Brinck, T., Murray, J.S., Politzer, P.: Quantitative analysis of molecular surfaces: areas, volumes, electrostatic potentials and average local ionization energies. J. Mol. Model. 16(11), 1679–1691 (2010)
Chang, H., Lee, J.D., Lee, S.M., Lee, Y.H.: Adsorption of NH3 and NO2 molecules on carbon nanotubes. Appl. Phys. Lett. 79, 3863–3865 (2001)
Chopra, N.G., Luyken, R.J., Cherrey, K., Crespi, V.H., Cohen, M.L., Louie, S.G., et al.: Boron nitride nanotubes. Science 269, 966–967 (1995)
Conley, D.J., Paerl, H.W., Howarth, R.W., Boesch, D.F., Seitzinger, S.P., Havens, K.E., Lancelot, C., Likens, G.E.: Controlling eutrophication: nitrogen and phosphorus. Science 323, 1014–1015 (2009)
Dai, J., Giannozzi, P., Yuan, J.: Adsorption of pairs of NOx molecules on single-walled carbon nanotubes and formation of NO + NO3 from NO2. Surface Sci. 603, 3234–3238 (2009)
Della Rocca, C., Belgiorno, V., Meriç, S.: Overview of in-situ applicable nitrate removal processes. Desalination 204, 46–62 (2007)
Deng, Z.-Y., Zhang, J.-M., Xu, K.-W.: Adsorption of SO2 molecule on doped (8, 0) boron nitride nanotube: a first-principles study. Physica E 76, 47–51 (2016)
Ditchfield, R., Hehre, W.J., Pople, J.A.: Self-consistent molecular-orbital methods. IX. An extended Gaussian-type basis for molecular-orbital studies of organic molecules. J. Chem. Phys. 54, 724–728 (1972)
Fewtrell, L.: Drinking-water nitrate, methemoglobinemia, and global burden of disease: a discussion. Environ. Health Perspect. 112, 1371–1374 (2004)
Ganesan, P., Kamaraj, R., Vasudevan, S.: Application of isotherm, kinetic and thermodynamic models for the adsorption of nitrate ions on graphene from aqueous solution. J. Taiwan Inst. Chem. Eng. 44, 808–814 (2013)
Hendricks, D., Water Treatment Unit Processes. Taylor and Francis Group, Boca Raton (2006)
Hesabi, M., Behjatmanesh-Ardakani, R.: Interaction between anti-cancer drug hydroxycarbamide and boron nitride nanotube: a long-range corrected DFT study. Comput. Theo. Chem. 1117, 61–80 (2017)
Hosseinian, A., Salary, M., Arshadi, S., Vessally, E.: The interaction of phosgene gas with different BN nanocones: DFT studies. Solid State Commun. 269, 23–27 (2018)
Jamshid, N.: Modulating band gap and HOCO/LUCO energy of boron-nitride nanotubes under a uniform external electric fiel. Iran. J. Chem. Chem. Eng. 36, 93–106 (2017)
Johnson, E.R., Keinan, S., Mori-Sanchez, P., Contreras-Garcia, J., Cohen, A.J., Yang, W.: Revealing noncovalent interactions. J. Am. Chem. Soc. 132, 6498–6506 (2010)
Kakemam, J., Noei, M.: Density functional study on the functionalization of BN nanotubes with nitramide. Russ. J. Phys. Chem. A. 88, 1751–1756 (2014)
Koswattage, K.R., Shimoyama, I., Baba, Y., Sekiguchi, T., Nakagaw, K.: ab Study on selective adsorption of deuterium on boron nitride using photon-stimulated ion-desorption. App. Surface Sci. 258, 1561–1564 (2011)
Mahdavifar, Z., Abbasi, N.: The influence of Cu-doping on aluminum nitride, silicon carbide and boron nitride nanotubes’ ability to detect carbon dioxide; DFT study. Physica E 56, 268–276 (2014)
Milmile, S.N., Pande, J.V., Karmakar, S., Bansiwal, A., Chakrabarti, T., Biniwale, R.B.: Equilibrium isotherm and kinetic modeling of the adsorption of nitrates by anion exchange Indion NSSR resin. Desalination 276, 38–44 (2011)
Mishra, P.C., Patel, R.K.: Use of agricultural waste for the removal of nitrate-nitrogen from aqueous medium. J. Environ. Manag. 90, 519–522 (2009)
Mossa Hosseini, S., Ataie-Ashtiani, B., Kholghi, M.: Nitrate reduction by nano-Fe/Cu particles in packed column. Desalination 276, 214–221 (2011)
Noei, M.: Different electronic sensitivity of BN and AlN nanoclusters to SO2 gas: DFT studies. Vacuum. 135, 44–49 (2017)
Noei, M., Ahmadaghaei, N., Salari, A.: A., Ethyl benzene detection by BN nanotube: DFT studies. J. Saudi Chem. Soc. 21, S12–S16 (2017)
O’Boyle, N., Tenderholt, A., Langner, K.: A library for package-independent computational chemistry algorithms. J. Comp. Chem. 29, 839–845 (2008)
Öztürk, N., Bektas, T.E.: Nitrate removal from aqueous solution by adsorption onto various materials. J. Hazard. Mater. B. 112, 155–162 (2004)
Paura, E.N.C., da Cunha, W.F., Martins, J.B.L., e Silva, G.M., Roncaratti, L.F., Gargano, R.: Carbon dioxide adsorption on doped boron nitride nanotubes. RSC Adv. 4, 28249–28258 (2014)
Rezaei-Sameti, M., Samadi Jamil, E.: The adsorption of CO molecule on pristine, As, B, BAs doped (4,4) armchair AlNNTs: a computational study. J. Nanostruct. Chem. 3, 1–9 (2016)
Rezaei-Sameti, M., Yaghoobi, S.: Theoretical study of adsorption of CO gas on pristine and AsGa-doped (4, 4) armchair models of BPNTs. Comput. Condens. Matter 3, 21–29 (2015)
Rezaei–Sameti, M., Moradi, F.: Interaction of isoniazid drug with the pristine and Ni-doped of (4, 4) armchair GaNNTs: a first principle study. J. Incl. Phenom. Macrocycl. Chem. 88, 209–218 (2017)
Rodriguez Juarez, A., Chigo Anota, E., Cocoletzi, H., Flores Riveros, A.: Adsorption of chitosan on BN nanotubes: a DFT investigation. App. Surface Sci. 268, 259–264 (2013)
Roohi, H., Maleki, L., Erfani Moradzadeh, M.: Exploring electronic properties and NO gas sensitivity of Si-doped SW-BNNTs under axial tensile strain. J. Mater. Sci. 52, 9739–9763 (2017)
Rubio, A., Corkill, J.L., Cohen, M.L.: Theory of graphitic boron nitride nanotubes. Phys. Rev. B 49, 5081 (1994)
Runge, E., Gross, E.K.U.: Density-functional theory for time-dependent systems. Phy. Rev. Lett. 52, 997–1000 (1984)
Samatya, S., Kabay, N., Yuksel, U., Arda, M., Yuksel, M.: Removal of nitrate fromaqueous solution by nitrate selective ion exchange resins. React. Funct. Polym. 66, 1206–1214 (2006a)
Samatya, S., Kabay, N., Yuksel, U., Arda, M., Yuksel, M.: Removal of nitrate from aqueous solution by nitrate selective ion exchange resins. React. Funct. Polym. 66, 1206–1214 (2006b)
Schmidt, M.W., Baldridge, K.K., Boatz, J.A., Elbert, S.T., Gordon, M.S., Jensen, J.H., Koseki, S., Matsunaga, N., Nguyen, K.A., Su, S.J., Windus, T.L., Dupuis, M., Montgomery, J.A.: General atomic and molecular electronic structure system. J. Comput. Chem. 14, 1347–1363 (1993)
Shao, P., Kuang, X.Y., Ding, L.P., Yang, J., Zhong, M.M.: Can CO2 molecule adsorb effectively on Al-doped boron nitride single walled nanotube? Appl. Surf. Sci. 285, 350–356 (2013)
Soltani, A., Raz, S.G., Rezaei, V.J., Khalaji, A.D., Savar, M.: Ab initio investigation of Al-and Ga-doped single-walled boron nitride nanotubes as ammonia sensor. Appl. Surf. Sci. 263, 619–625 (2012)
Soltani, A., Ahmadi Peyghan, A., Bagheri, Z.: H2O2 adsorption on the BN and SiC nanotubes: a DFT study. Physica E 48, 176–180 (2013)
Tabtimsai, C., Nonsri, A., Gratoo, N., Massiri, N., Suvanvapee, P., Wanno, B.: Carbon monoxide adsorption on carbon atom doped perfect and Stone–Wales defect single-walled boron nitride nanotubes: a DFT investigation. Monatsh Chem. 145, 725–735(2014)
Tontapha, S., Ruangpornvisuti, V., Wanno, B.: Density functional investigation of CO adsorption on Ni-doped single-walled armchair (5, 5) boron nitride nanotubes. J. Mol. Model. 19, 239–245 (2013)
Wang, C., Guo, C.: The noble gases adsorption on boron-rich boron nitride nanotubes: a theoretical investigation. Superlat. Microstr. 107, 97–103 (2017)
Wang, R.X., Zhang, D.J.: Theoretical study of the adsorption of carbon monoxide on pristine and silicon-doped boron nitride nanotubes. Aust. J. Chem. 61, 941–945 (2008)
Wang, R.X., Zhang, D.J., Liu, C.B.: The germanium-doped boron nitride nanotube serving as a potential resource for the detection of carbon monoxide and nitric oxide. Comput. Mater. Sci. 82, 361–366 (2014)
Wang, R., Zhang, D., Liu, C.: DFT study of the adsorption of 2,3,7,8-tetrachlorodibenzo-p-dioxin on pristine and Ni-doped boron nitride nanotubes. Chemosphere 168, 18–24 (2017)
Wehling, T.O., Noveselov, K.S., Morozov, S.V., Vdovin, E.E., Katsnelson, M.I., Geim, A.K., Lichtenstein, A.I.: Molecular doping of graphene. Nano Lett. 8, 173–177 (2008)
Xie, Y., Huo, Y.P., Zhang, J.M.: First-principles study of CO and NO adsorption on transition metals doped (8, 0) boron nitride nanotube. Appl. Surf. Sci. 258, 6391–6397 (2012)
Xing, X., Gao, B.-Y., Zhong, Q.-Q., Yue, Q.-Y., Li, Q.: Sorption of nitrate onto amine-crosslinked wheat straw: characteristics, column sorption and desorption properties. J. Hazard. Mater. 186, 206–211 (2011)
Yim, W.L., Gong, X.G., Liu, Z.F.: Chemisorption of NO2 on carbon nanotubes. J. Phys. Chem. B. 107, 9363–9369 (2003)
Zhang, M.L., Ning, T., Zhang, S.Y., Li, Z.M., Yuan, Z.H., Cao, Q.X.: Response time and mechanism of Pd modified TiO2 gas sensor. Mater. Sci. Semicond. Process. 17, 149–154 (2014)
Acknowledgements
The author thanks the Computational information center of Malayer University for providing the necessary facilities to carry out the research.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rezaei-Sameti, M., Zarei, P. NBO, AIM, HOMO–LUMO and thermodynamic investigation of the nitrate ion adsorption on the surface of pristine, Al and Ga doped BNNTs: A DFT study. Adsorption 24, 757–767 (2018). https://doi.org/10.1007/s10450-018-9977-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-018-9977-7