Abstract
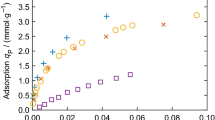
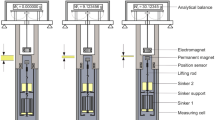
Determining sample volumes for gas sorption measurements by pycnometry is often considered to be trivial, as it is assumed that helium does not sorb to the sample material. The aim of this study is to show the contrary, i.e., helium may sorb to certain polymers and to introduce the proposed helium-sorption correction algorithm for compensating for such helium sorption. By using a Rubotherm magnetic suspension balance, the volume of a commercial polyether block amide (Pebax© MH 1657) was analyzed with the new algorithm based on kinetic data. Helium was found to sorb to all tested Pebax© MH 1657 samples. Compared to the conventional calculations, the proposed algorithm yielded more precise sample volumes. To ensure accurate sorption measurements of any gas, it is important to take into account possible helium sorption.









Similar content being viewed by others
References
Aston, J.G., Mastrangelo, S.V.R.: The anomalous first layer of adsorbed helium and a modified b.e.t. theory. J. Chem. Phys. 19(8), 1067–1068 (1951)
Aston, J.G., Mastrangelo, S.V.R., Tykodi, R.J.: Adsorption of helium on titanium dioxide. J. Chem. Phys. 23(9), 1633–1635 (1955)
Belmabkhout, Y., Frere, M., De Weireld, G.: Adsorption equilibria of single gas and gas mixture on homogeneous surfaces: a unified approach based on statistical thermodynamics developments. part i: single gas adsorption. Mol. Simul. 32(7), 495–502 (2006)
Bondar, V.I., Freeman, B.D., Pinnau, I.: Gas sorption and characterization of poly(ether-b-amide) segmented block copolymers. J. Polym. Sci. Pol. Phys. 37(17), 2463–2475 (1999)
Car, A., Stropnik, C., Yave, W., Peinemann, K.V.: Peg modified poly(amide-b-ethylene oxide) membranes for co2 separation. J. Membr. Sci. 307(1), 88–95 (2008)
Condon, J.B.: Surface Area and Porosity Determinations by Physisorption—Measurements and Theory. Elesevier B.V. (2006)
Fleming, G.K., Koros, W.J.: Dilation of polymers by sorption of carbon dioxide at elevated pressures. 1. silicone rubber and unconditioned polycarbonate. Macromolecules 19(8), 2285–2291 (1986)
Gumma, S., Talu, O.: Gibbs dividing surface and helium adsorption. Adsorption 9, 17–28 (2003)
Keller, J., Dreisbach, F., Rave, H., Staudt, R., Tomalla, M.: Measurement of gas mixture adsorption equilibria of natural gas compounds on microporous sorbents. Adsorption 5,199–214 (1999)
Keller, J.U., Robens, E.: A note on sorption measuring instruments. J. Therm. Anal. Calorim. 71, 37–45 (2003)
Keller, J.U., Staudt, R.: Gas Adsorption Equilibria—Experimental Methods and Adsorptive Isotherms. Springer Science + Business Media, Inc. (2005)
Kim, J.H., Lee, Y.M.: Gas permeation properties of poly(amide-6-b-ethylene oxide)-silica hybrid membranes. J. Membr. Sci. 193(2), 209–225 (2001)
Maggs, F.A.P., Schwabe, P.H., Williams, J.H.: Adsorption of helium on carbons: influence on measurement of density. Nature 186(4729), 956–958 (1960)
Marcq, J., Nguyen, Q.T., Langevin, D., Brule, B.: Abatement of co2 emissions by means of membranes - characterization of industrial pebax films. Environ. Prot. Eng. 3–4(3-4), 13–22 (2005)
Mastrangelo, S.V.R., Aston, J.G.: Thermodynamic data and some notes on the nature of adsorbed helium. J. Chem. Phys. 19(11), 1370–1375 (1951)
Robens, E., Keller, J., Massen, C., Staudt, R.: Sources of error in sorption and density measurements. J. Therm. Anal. Calorim. 55, 383–387 (1999)
Sircar, S.: Measurement of gibbsian surface excess. AIChE J. 47(5), 1169–1176 (2001)
Sircar, S.: Role of helium void measurement in estimation of gibbsian surface excess. In: Fundamentals of Adsorption, vol. 7, pp. 656–663. Japan, (2002)
Springer, C., Major, C.J., Kammermeyer, K.: Low-pressure adsorption of helium on microporous solids. J. Chem. Eng. Data 14(1), 78–82 (1969)
Staudt, R., Bohn, S., Dreisbach, F., Keller, J.U.: Gravimetric and volumetric measurements of helium adsorption equilibria on different porous solid. In: Mc Ennany ea (ed) Proceedings COPS IV Conference, Bath, Sept. 1996, The Royal Society of Chemistry, Special Publ. No. 213, pp. 261–266. London (1997)
Steele, W.A., Halsey, G.D.: The interaction of rare gas atoms with surfaces. J. Chem. Phys. 22(6), 979–984 (1954)
Suzuki, I., Kakimoto, K., Oki, S.: Volumetric determination of adsorption of helium over some zeolites with a temperature-compensated, differential tensimeter having symmetrical design. Rev. Sci. Instrum. 58(7), 1226–1230 (1987)
Talu, O., Myers, A.L.: Molecular simulation of adsorption: Gibbs dividing surface and comparison with experiment. AIChE J. 47(5), 1160–1168 (2001)
Vopicka, O., Hynek, V., Zgazar, M., Friess, K., Spek, M.: A new sorption model with a dynamic correction for the determination of diffusion coefficients. J. Membr. Sci. 330(1-2), 51–56 (2009)
Acknowledgments
We would like to thank the Alexander von Humboldt Foundation for financial support of this research endeavor.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lorenz, K., Wessling, M. How to determine the correct sample volume by gravimetric sorption measurements. Adsorption 19, 1117–1125 (2013). https://doi.org/10.1007/s10450-013-9537-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-013-9537-0