Abstract
This paper reviews the 40-year evolution and application of the magnetic suspension balance (MSB) and discusses some challenging issues of the technique. An MSB, as defined herein, is a magnetic suspension coupling (MSC) connected to an analytical balance. With an MSC, an object can be weighed in a different environment than the balance itself, making it possible for contactless weighing. Over the past 40 years, the MSB has been commonly used in research areas requiring accurate object weighings, notably gas density measurements by MSB-based densimeters and gas adsorption measurements by MSB-based sorption analyzers. More than 15 MSB-based densimeters have been built to date; these are generally called two-sinker densimeter and single-sinker densimeter. They have produced highly accurate density data of many pure fluids and fluid mixtures. These data serve as the basis for the development of reference equations of state, which play an essential role in various industrial and scientific areas. Moreover, such systems are central to the metrology program of many countries. The MSB technique is also very successful in adsorption science: more than 85 MSB-based sorption analyzers have been set up in over 20 countries. The number of new MSB-based sorption analyzers, and peer-reviewed publications resulting from them, are both increasing exponentially since 2004. They have produced highly reliable gas adsorption data at high pressures for many applications, mainly in the energy and environmental sectors. Although further development of innovative instruments based on the MSB is threatened by the proprietary nature of MSB technology, the development will continue, e.g., toward cryogenic measurements and a more compact design.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
A magnetic suspension balance (MSB) is a magnetic suspension coupling (MSC) connected with an (commercial) analytical balance. Here, the MSC is the key component that makes it possible for contactless weighing—in other words, it allows the weighing of an object in a different environment than the balance itself. Over the past 40 years, the MSB has been commonly used in research areas requiring accurate object weighings. Most notably, these include densimetry (i.e., determination of fluid density over wide ranges of temperature and pressure by application of the Archimedes’ buoyancy principle) and sorption science (i.e., determination of the quantity of a gas adsorbed onto the surface of a solid object). Other applications include thermogravimetry, chemical reactions, absorption, diffusion effects, and viscometry. The essential elements of an MSB are two magnets, one on each side of a wall separating the measuring environment and the balance environment. An electromagnet with an iron core hangs from the balance and attracts a permanent magnet inside the measuring space, which is held in a freely suspended state. The object to be weighed, in turn, is attached to the permanent magnet. Also necessary are a position sensor and a feedback control loop that makes fine, continual adjustments to the current in the electromagnet to maintain the permanent magnet + object in a stable suspension. Thereby, the weight of the object is transmitted to the balance.
In this review article, we carry out a comprehensive review of the MSB technique, especially focusing on its applications to densimetry and sorption science. Section 2 considers the evolution of the different embodiments of this technology. For many years, both areas have been part of the research portfolio of Professor Roland Span, to whose 60th birthday this paper is dedicated. Sections 3 and 4 focus on the application in densimetry and sorption science, respectively. Section 5 considers the measurement uncertainties, especially focusing on the force transmission error (FTE), which is unique to MSB instruments, and Sect. 6 outlines recent challenges to be resolved. The main purpose of this review is to present a brief history (evolution and application), summarize key information (measuring principle and uncertainty), discuss current challenges, and propose future developments for the MSB technique.
2 Evolution of the Magnetic Suspension Balance Technology
This section describes the evolution of the MSB technology. An overview of the evolution is given in Fig. 1, where the key developers, milestones, and critical information of the evolution are given briefly. More details are presented in the following subsections.
2.1 Early Systems
The invention and evolution of the MSB technique was originally motivated by the desire for highly accurate fluid density data. The earliest system for measuring liquid density that employed an electromagnet to measure forces was developed by Lamb and Lee in 1913 [1]. Later, a variety of other systems were developed from the 1940s through the 1980s, e.g., densimeters employing magnetic levitation as developed by Beams et al. [2], Beams and Clarke [3], Haynes et al. [4], Masui et al. [5, 6], and Wolf et al. [7]. In these devices, a sinker (sometimes called a buoy) was held in a suspended state by means of magnets. These instruments, however, do not fall within the scope of the MSB as we define it here; they were described in more detail in a review article by McLinden [8].
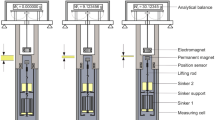
The principle of contactless weighing was first developed by Clark in 1947 [9]. The first type of a magnetic suspension coupling (MSC) was developed by Gast and presented in 1962 [10]. In conjunction with a conventional beam balance, it was first referred to as ‘MSB.’ The version presented by Gast in 1967 and 1969 [11, 12] is shown in Fig. 2. This type was commercially available from Sartorius AG, Germany,Footnote 1 until the 1980s. The equipment was developed for weighing in closed vessels, which may be either evacuated or filled with highly reactive gases, where the balance was located outside the vessel.
Schematic diagram of the ‘magnetic suspension balance’ (Type 4201) from Sartorius AG. It was developed by Gast and presented in 1962 [10] and also described in [11, 12]. The distance between the electromagnet and the suspended magnet was controlled by means of a position sensor and a control winding in the upper magnet
The operating principle of the MSC presented by Gast in 1967 and 1969 [11, 12] was as follows: An upper magnet, connected to a conventional beam balance, interacted with a lower magnet (suspended magnet) inside the closed vessel. This interaction kept the lower magnet and the object attached to it in free suspension. In this way, the suspended load was transmitted without contact from the lower magnet through the wall of the vessel to the upper magnet. The upper magnet (electromagnet) consisted of a permanent magnet, an iron casing, a control winding, and a position sensor coil. The lower magnet consisted of a permanent magnet, an iron casing, and a copper disc on its top. Furthermore, the distance between the electromagnet and the suspended magnet was indicated by the position sensor. Independent of the suspended load, the distance between the two magnets was adjusted via a control circuit in such a way that the current through the control winding in the upper magnet was driven to near zero. This means that the lower magnet was supported by the upper one with almost no additional magnetic field from the control winding. A small current was only needed to keep a stable position of the suspended magnet. Hence, practically no heat was generated in this coil, a fact which was very important for the accuracy of the balance. Due to the measuring principle of the position sensor, the material of the separating wall between the two magnets had to be made of an electrically non-conductive material (dielectric window), e.g., glass. For higher pressures up to a few MPa, an autoclave could be used instead of the measuring cell of glass, and a sapphire window could be used as separating wall. For thermogravimetric analyses, a special autoclave in conjunction with an ‘MSB’ from Sartorius was developed by Sabrowsky and Deckert [13, 14]; the permissible operating pressure was given as 30 MPa. This was achieved by installing a special window (as a separating wall) made of electrically non-conductive and non-magnetic material; the type of material was not specified by the authors. (In the dissertation of Lösch [15], on page 41, a single-crystal sapphire was mentioned as the material.)
2.2 Single-Position MSB
In the early 1980s, a novel two-sinker densimeter was developed by Kleinrahm [16] that implemented two key innovations. The first innovation was to completely separate the MSC from the weighing device; this allowed the use of commercial analytical balances and their extraordinary accuracy and simultaneously allowed much greater flexibility in the design of the measuring cell. The second innovation was the use of two sinkers, that were of the same mass but very different volumes, as the suspended load. Moreover, by fabricating the two sinkers to have the same surface area and same surface material, adsorption effects of gases on the sinker surfaces were greatly reduced, as discussed in Sect. 3.3. This two-sinker principle is a very accurate differential (compensation) method, which largely reduced the influence of various possible sources of errors. For weighing the load of the sinkers in a closed measuring cell, a modified version of Gast’s MSC was used; it was developed by Gast, Kleinrahm, Lösch, and Wagner. Figure 3 shows a schematic drawing of this apparatus. This new densimeter and the first measurement results were published by Kleinrahm and Wagner [17, 18].
The operating principle of this MSC was the same as explained above, but the shape of the two magnets was adapted to the new application. For weighing the suspended load in the measuring cell, a commercial analytical balance from Sartorius was used (type: 2004 MP6, weighing range: 166 g, electrical weighing range: 16 g, readability: 0.00001 g). This balance was located at ambient environment. For separating the permanent magnet in the pressurized measuring cell from the electromagnet at ambient pressure, a glass tube (outer diameter: 22 mm, thickness: 1.2 mm, material: Duran 50) with a hemispherical shape at the bottom was used; the permissible pressure was 12 MPa. When the electromagnet was switched off, the permanent magnet with its suspended assembly was held by a support.
This novel densimeter was specially developed for accurate measurements of saturated-liquid and saturated-vapor densities of pure fluids. The temperature range covered was T = (50 to 350) K at pressures up to 8 MPa; for liquids, the expanded uncertainty (k = 2) in density was 0.02%. A novel differential method was applied that was based on Archimedes’ buoyancy principle. Two sinkers of identical mass and surface area but with a considerable difference in volume were used. One of the sinkers was a gold-plated quartz-glass sphere (VS ≈ 24.5 cm3, mS ≈ 54 g) and the other was a disc of solid gold (VD ≈ 2.8 cm3, mD ≈ 54 g). The sinkers could be put alternately on a sinker support or lifted from it by means of a sinker-changing device. The sinker support was connected to the permanent magnet by a thin wire and, via the MSC, to the analytical balance. This was a “single position” MSB, i.e., either “Off” or measuring position “MP.” The Off position was used to change the sinkers (see Sect. 3.2). The “MP” position of the sphere was used as tare position, i.e., the balance was tared to 0.00000 g with the sphere in suspension. For a density measurement the sinkers were usually exchanged 10 to 30 times, so that the weighing difference of the two sinkers, surrounded by the fluid, could be measured. Thus, the density ρ of the fluid in the measuring cell could be determined by the simple basic equation ρ = (mD − mS)/(VS − VD), where the subscripts D and S refer to the disk and sphere. The MSC initially used was replaced a few years later by a new MSC type with two positions (see Sect. 2.3). Comprehensive measurements of many fluids were carried out with this densimeter, as summarized in Table 1 in Sect. 3.1.
2.3 MSB with Two Positions
Lösch achieved a decisive advance in MSB technology in 1987 [15]. The core innovation was a different type of position sensor. This innovation allowed its application to density measurements at extreme conditions and with high accuracy. Moreover, this new MSC type was used successfully for a variety of other sophisticated gravimetric applications. Typical versions of the new MSC type and their applications were briefly presented by Lösch et al. [19, 20].
Three significant modifications characterize this 1987 embodiment of the MSC. (1) Instead of a distance measurement between the electromagnet and the permanent magnet, only the vertical position of the permanent magnet relative to the coupling housing (see Fig. 4 which schematically shows a possible arrangement of this MSC) was determined, and its position was controlled in a direct loop, independent of the distance to the electromagnet. Hence, this position sensor could now be placed at a convenient position. For most applications, a moving coil sensor was an appropriate sensor; it consisted of a sensor core (high-frequency ferrite core), which was located inside the coupling housing, and a sensor coil, that was located outside the coupling housing. (2) The use of the new position sensor resulted in another decisive advantage; the only limitation on the material used for the separating wall between the two magnets is that it should be as non-magnetic as possible. For example, a coupling housing made of the copper-beryllium alloy CuBe2 is only slightly diamagnetic, and that caused only a very small, so-called “force transmission error”; this effect is explained in Sect. 5.2. Due to the possibility of using metals for the coupling housing, pressures of up to 100 MPa could now be realized in the measuring cell. (3) By using the new position sensor, which enabled a relatively large measuring range, two weighing positions could be realized: a zero or tare position (ZP or TP), where the balance could be tared and calibrated, and a weighing or measuring position (MP), where a measuring load could be connected and weighed. This is schematically represented in Fig. 4. The position of the permanent magnet is detected by a position sensor and controlled in a direct and fast loop (PID controller). By means of a superimposed set-point controller and an additional control system, several vertical motions of the permanent magnet are generated automatically. In this way, soft upward and downward movements of the permanent magnet could be realized, and via the load coupling and decoupling device the measuring load could be coupled and decoupled. In both positions, the lower magnet is entirely lifted by the iron core of the upper magnet, and only a small current in the control winding of the upper magnet is needed to control and keep a stable position of the suspended magnet.
Schematic representation of the “OFF” position and the two weighing positions of a typical commercial gravimetric sorption analyzer. OFF: In this position, the electromagnet is switched off and the permanent magnet with the lifting rod assembly is placed on its base; ZP (TP): zero position or tare position, where only the permanent magnet with the lifting rod assembly is in suspension; MP: measuring position, where the container with its lifting rod and the adsorbent sample inside are lifted. (Analogous to Lösch [15] and Lösch et al. [19, 20])
This type of MSB enabled a variety of applications covering large ranges of temperature and pressure, e.g., densimetry, sorption measurements, and thermogravimetric analyses. One type of such equipment implementing an MSB is the so-called “single-sinker densimeter,” which is now a very important and well-established method for accurate density measurements of fluids.
2.4 MSB with Three Positions
A further refinement of the two-position MSB of Lösch [15] was presented by Dreisbach and Lösch [21]. Here, there are two measuring positions (MP1 and MP2) in addition to the zero position (ZP). This highly sophisticated type of MSB was especially designed for the simultaneous measurement of adsorption equilibria of gases on the surface of porous material, as well as the density of the gas. For this purpose, three weighing positions were realized with this type of MSC; this is shown in Fig. 3 of the paper of Dreisbach and Lösch [21]. Here, a schematic arrangement is shown in Fig. 5. This apparatus and its application is described in detail in reference [21]. Another application, where the adsorbent sample and its container is replaced by several thin, non-porous metal sheets was presented by Kleinrahm et al. [22] and Yang and Richter [23] and named a “tandem-sinker densimeter.”
Schematic representation of the three weighing positions of a commercial gravimetric sorption analyzer. ZP (TP): zero position or tare position, where only the permanent magnet with the lifting rod assembly is in suspension; MP1: measuring position 1, where the container with its lifting rod and the adsorbent sample inside are lifted; MP2: measuring position 2, where the density sinker at the top position is lifted into suspension as well. For OFF position see Fig. 4. (Analogous to Dreisbach and Lösch [21])
2.5 Commercial Availability
Since 1990, MSBs have been commercially produced and marketed by Rubotherm GmbH, Germany. A large variety of special instruments with integrated MSBs have also been available, e.g., for fluid density measurement, sorption measurement, and thermogravimetric analyses. Since 2016 the company is a subsidiary of TA Instruments, USA. For a number of years, MSBs and instruments with integrated MSBs have also been available from Rubolab GmbH, Germany and Linseis Messgräte GmbH, Germany. There are also several high-accuracy gravimetric sorption analyzers produced by Hiden Isochema, UK and used worldwide; these instruments incorporate an analytical balance and use magnets; however, they use a beam rather than an MSC as a lift mechanism. Therefore, the products of Hiden Isochema are not considered in this review.
3 Application in Densimetry
3.1 List of MSB-Based Densimeters
A review of MSB-based densimeters for accurate density measurements of fluids over large ranges of temperature and pressure before 2004 was given by Wagner and Kleinrahm [24]. Since then, additional MSB-based densimeters were developed, mainly by Rubotherm. A list of single-sinker densimeters in use in 2015 was given by Yang et al. [25]; starting with this review, a more comprehensive review of MSB-based densimeters (including single-sinker densimeters, two-sinker densimeters, and special densimeters, as well as both active and decommissioned instruments) are given in Table 1 together with the measured fluids.
3.2 Sinkers
For density measurements employing any type of Archimedes’ technique, including MSB instruments, the sinker is at the core of the measurement. The mass and volume of the sinker enter directly into the calculation for density (e.g., see Sect. 3.3) and must be known accurately over the full temperature and pressure range of the instrument. The uncertainties in the sinker parameters, together with the weighing uncertainties, dominate the overall density uncertainty obtainable with an MSB instrument, as discussed in Sect. 5.
A sinker must satisfy a number of requirements. First of all, it must be more dense than the surrounding fluid so that it sinks, rather than floats. (The entire Archimedes’ technique could be inverted to employ “floats” with a density lower than the fluid, with the MSB providing a downward force, as done by Lamb and Lee in [1], but this approach is rarely taken nowadays.) A relatively low-density sinker provides a greater buoyancy force and thus greater sensitivity for density. The sinker must be chemically stable in the fluid—any corrosion would change its mass and/or volume. The change of sinker volume with temperature and pressure is most commonly calculated from materials properties, and thus the coefficient of thermal expansion and the bulk modulus of elasticity should be well characterized.
Given these requirements, the sinker materials often used are single-crystal silicon, fused quartz (also called quartz glass), and titanium. Silicon, with a density of approximately 2330 kg·m–3, is somewhat more dense that almost all common liquids; its main advantage is that the physical properties are known very accurately, and because it is readily available as single-crystal material in ultra-high purities, literature values of the thermal expansion coefficient [129] can be applied. However, silicon is readily oxidized and this limits its use. Fused quartz has a density of approximately 2200 kg·m–3, which is similar to silicon, and it is much more corrosion resistant. Although the thermal expansion coefficient of quartz is not known as well as that of silicon, it is much lower, and this reduces the uncertainty of the sinker volume with changes in temperature. Titanium with a density of approximately 4510 kg·m–3 is a medium-density metal and is corrosion resistant. Hollow metal sinkers have been employed by some authors [46,47,48] to combine the corrosion resistance of, for example, stainless steel with a low density. A concern here is the volume can be a strong function of pressure, and hollow sinkers are typically used only at relatively low pressures.
Sinkers have been fabricated in a large variety of geometric forms. The most typical form is illustrated in Fig. 6, which a titanium sinker used in the tandem-sinker densimeter (see Table 1) at Chemnitz University of Technology (TUC). Metals are easier to fabricate in complex forms; silicon and quartz are fabricated by grinding, and this limits the possibilities.
Photo of a typical sinker. [23]
The sinkers in a two-sinker densimeter must be of different densities, and maximizing the difference increases the sensitivity of the measurement. Kleinrahm and Wagner [18] used quartz and gold; McLinden and Lösch-Will [55] used titanium and tantalum; Kayukawa et al. [67] used silicon and germanium, and Pieperbeck et al. [46, 47] as well as Richter et al. [48] used stainless steel for both sinkers with one being hollow. Gold and tantalum are high-density metals (ρAu = 19,250 kg·m–3 and ρTa = 16,690 kg·m–3); both are highly corrosion resistant.
Most measurements with an MSB (e.g., a two-sinker densimeter and a gravimetric sorption analyzer to be discussed in Sect. 4.2) require two or more distinct weighings, and this requires some means to switch between sinkers or adsorbent samples being weighed. Various mechanisms have been implemented. The simplest and most common changing mechanism makes use of the vertical motion of the permanent magnet as it moves between ZP and one or more measuring positions (see Sect. 2.3 and 2.4). Alternatively, the two-sinker densimeter developed by Kleinrahm and Wagner [16,17,18] and the four-sinker densimeter developed by Moritz, Kleinrahm, McLinden, and Richter [127, 128] use an external actuator; the two-sinker densimeter developed by McLinden and Lösch-Will [55] uses a rotary mechanism.
3.3 Two-Sinker Densimeters
The accuracy of the Archimedes’ technique, especially at low densities, can be improved by the use of two sinkers. This technique was first developed by Kleinrahm and Wagner [16,17,18] in the 1980s (see Sect. 2.2). For the two-sinker technique, the density is given by
where the subscripts refer to the two sinkers, which (usually) have the same mass, same surface area, and same surface material, but very different volumes (either by use of different materials or by employing solid and hollow sinkers of the same material). Systematic errors, including gas adsorption on the sinker surface, balance non-linearity, and the influence of magnetic materials on the coupling are greatly reduced by the use of two sinkers and the resulting differential weighing.
Although the development of the two-sinker instrument predates the single-sinker instruments described in Sect. 3.4, only a handful of two-sinker instruments have been built to date (see Table 1). The greater accuracy of the two-sinker approach compared to a single-sinker instrument comes at the expense of greater complexity, and many applications can be satisfied by a simpler single-sinker instrument.
The two-sinker densimeter developed at the Ruhr-Universität Bochum (RUB) by Kleinrahm and Wagner [16,17,18] represented a major advance in density measurement over wide ranges of temperature and pressure. The core of the system is illustrated in Fig. 3. It operated at temperatures from 60 K to 340 K, with pressures to 12 MPa; the density range was 1 kg·m–3 to 2000 kg·m–3. The sinkers were a gold-plated quartz sphere (m = 54 g, V = 24.5 cm3) and a gold disk (m = 54 g, V = 2.8 cm3). These were alternately placed on the “sinker support” for weighing by a mechanism involving a small winch outside the main thermostat (but immersed in the fluid), which actuated wires connected to devices which lowered the sinkers onto the sinker support. The MSC was separated from the measuring cell, and it was thermostated near ambient temperature. The total experimental uncertainty of this instrument was 0.01% to 0.02%, depending on the measurement conditions. It has been used to measure many fluids, as listed in Table 1. The resulting data were key for the development of reference equations of state for these fluids, as discussed in Sect. 3.6.
The two-sinker densimeter of Pieperbeck et al. [46, 47] employs the same type of coupling and sinker-changing mechanism as the instrument of Kleinrahm and Wagner [16,17,18]. It was designed primarily for the measurement of natural-gas mixtures and operated over the temperature and pressure range of natural-gas pipelines, namely 273 K to 323 K, with pressures to 12 MPa; the density range was 1 to 1000 kg·m–3. The sinkers were a hollow sphere (m = 123 g, V = 107 cm3) and a solid ring (m = 123 g, V = 15.6 cm3); both were of stainless steel with electro-polished and gold-plated surfaces. The large volume of the hollow sinker increased the sensitivity for low-density gas phase measurements. In this context, a special two-sinker densimeter should also be mentioned [48], which does not use an MSC. It was developed for the gas industry to measure the density of natural gases at very low densities at standard conditions (T = 273.15 K, p = 0.101325 MPa). The two sinkers are a hollow cylinder (V ≈ 500 cm3, m ≈ 200 g) and a solid triple ring (V ≈ 25 cm3, m ≈ 200 g) made of stainless steel; the surfaces (A ≈ 360 cm2) of both sinkers are gold plated. The total measurement uncertainty is given as 0.02% in density.
The two-sinker densimeter at the National Institute of Standards and Technology (NIST) described by McLinden and Lösch-Will [55] combines the advantages of the two-sinker technique with those of the compact design of many single-sinker instruments (see Sect. 3.4). It operates over the temperature range from 210 K to 505 K with pressures to 40 MPa. The sinkers are made of titanium (m = 60 g, V = 13.3 cm3) and tantalum (m = 60 g, V = 3.6 cm3). Sinker changing is accomplished by a mechanism that rotates the electromagnet, which in turn induces a matching rotation of the permanent magnet; two sets of “lifting forks” attached to the permanent magnet engage “pins” on the sinkers (see Fig. 7). The motor operating this mechanism is located in ambient air, i.e., not in the measuring cell like earlier designs; this largely avoids moving parts in the measuring cell and possible condensation of gas in a “remote” mechanism. This densimeter also employs external masses placed on the balance pan, and while these are very similar to the compensation masses used with single-sinker densimeters (see Sect. 3.4). They are used instead to calibrate the balance as a part of each density measurement and in the determination of the force transmission error (see Sect. 5.2). The weighing design for this instrument involves two weighings of each of the four objects (two sinkers and two external masses) in a time-symmetric design that largely cancels any small drift in the temperature or pressure in the measuring cell [61]. This instrument has uncertainties ranging from 0.004% at near-ambient conditions to 0.02% at the extremes of temperature and pressure [130]. It has been used to measure both pure fluids and mixtures, as listed in Table 1. It has also been used to investigate adsorption effects, especially near mixture dew points [64, 131]; this was preparatory work for the four-sinker densimeter described in Sect. 3.5.2.
Sinkers and magnetic suspension coupling in the densimeter of McLinden and Lösch-Will. [55] The electromagnet at the top hangs from the balance and levitates the permanent magnet below it. In this photo, the bottom (titanium) sinker is being weighed, while the top (tantalum) sinker sits on its rest
Kayukawa et al. [67] at the National Metrology Institute of Japan describe a two-sinker system with sinkers of single-crystal silicon (m = 61 g, V = 26.2 cm3) and single-crystal germanium (m = 60 g, V = 11.3 cm3). The temperature range is 278 K to 323 K, with pressures to 20 MPa. An uncertainty in density of 1 part in 106 (not including uncertainties associated with the temperature and pressure measurement) is anticipated from this system due to the combination of single-crystal sinkers (which are very stable and well-characterized materials), a very detailed finite-element analysis of the force transmission error, and a coupling that maintains a constant position of the permanent magnet for the different weighings (as opposed to maintaining zero-current in the electromagnet, as is the case for most existing systems). Thus far, preliminary results with tridecane at T = 273.15 K have yielded an uncertainty of 0.0005%. No further publications on this instrument have appeared in literature.
3.4 Single-Sinker Densimeters
The main feature of the “two-sinker method,” namely, very high accuracies especially in the low gas-density range, is of less importance for many scientific and technical applications. Compared to two-sinker densimeters, single-sinker densimeters are much simpler in construction, easier to use, and simplify the density measurement, especially for medium and high densities.
3.4.1 Measurement Principle
Based on the MSB with two positions (see Sect. 2.3), a new type of densimeter, called a “single-sinker densimeter,” was developed by Brachthäuser, Kleinrahm, Lösch, and Wagner in the early 1990s [68, 69]. It is based on Archimedes’ buoyancy principle applied in a special way; its principle is roughly illustrated in Fig. 4, where a sinker replaces the sample container shown. A more detailed schematic can be seen in Fig. 2 of the paper of Wagner et al. [69] and Fig. 7 of the paper Wagner and Kleinrahm [24]. In the system of Brachthäuser et al. [68, 69], the sinker, a cylinder of quartz glass (\({V}_{\text{S}}\) ≈ 26.5 cm3, \({m}_{\text{S}}\) ≈ 60 g, \({\rho }_{\text{S}}\) ≈ 2200 kg·m–3), was contained in a pressure-proof measuring cell. For density measurements, the sinker was connected to a commercial analytical balance (Mettler AT 201, weighing range 205 g, resolution 0.01 mg) via an MSC. The density of the fluid inside the measuring cell can be determined by the simple equation
where \({m}_{\text{S}}\) is the ‘true’ mass of the sinker, \({m}_{\text{S}}^{*}\) is the ‘apparent’ mass of the sinker when surrounded by the fluid, and Vs is its volume, which depends on temperature and pressure. The mass of the sinker, \({m}_{\text{S}}\), is accurately determined by weighing in the evacuated measuring cell. For a density measurement, the positions TP and MP (see Fig. 4) were changed several times, and an average value, \({m}_{\text{S}}^{*}\), was calculated from the readings. The pressure-proof coupling housing is made of beryllium copper, a magnetically neutral metal. The basic design of the single-sinker densimeter is shown in Fig. 3 of the paper of Wagner et al. [69] and in Fig. 8 of the paper Wagner and Kleinrahm [24]. The initial version of this single-sinker densimeter was improved in the following years by Klimeck et al. [70]. With this improved system, the density of many technically or scientifically important pure fluids have been comprehensively measured (see RUB III in Table 1). A summarized description of single-sinker densimeters can be found in reference [24].
The sinker volume, Vs, should be determined as accurately as possible. Its temperature and pressure dependence is given by a typical solid volume expansion equation [25]:
\({V}_{0}\) is the volume of the sinker at reference state (e.g., T0 = 293.15 K and p0 = 0.10135 MPa), \({\stackrel{-}{\alpha}}|_{{T}_{0}}^{T}\) is the average value of the linear thermal expansion coefficient α(T) in the temperature range from T0 to T, and K(T) is the isothermal compression modulus. Single-crystal silicon is recommended as the ‘best’ material of a sinker because its α(T) and K(T) values are accurately known. In the temperature range from (293 to 1000) K, α(T) for single-crystal silicon was given by Watanabe et al. [129] with the uncertainty impact on the value of VS being less than 7 ppm. In the temperature range from (298.15 to 423.15) K, K(T) can be calculated by
where c11(T) and c12(T) are elastic moduli, values of which can be obtained from [132, 133]. For the calculation of the temperature and pressure dependence of the volume, \({V}_{\text{S}}\left(T,p\right)\), of a single-crystal silicon sinker, two simple equations are given in the Appendices A2 and A3 of [118].
3.4.2 Compensation Masses
To achieve a high level of accuracy with the single-sinker densimeter, especially at relatively low densities, the analytical balance is only operated close to its tare point using a basic load compensation mechanism [69, 134] (see principle in Fig. 2 of [69] and Fig. 7 of [24] as well as the photo in Fig. 8). In the tare position, a tantalum weight (\({m}_{\text{Ta}}\) ≈ 82 g, \({V}_{\text{Ta}}\) ≈ 4.9 cm3, \({\rho }_{\text{Ta}}\) ≈ 16,700 kg·m–3 [69]) is placed on the balance. When switching to the measuring position, the tantalum weight is exchanged with a weight made of titanium (\({m}_{\text{Ti}}\) ≈ 22 g, \({V}_{\text{Ti}}\) ≈ 4.9 cm3, \({\rho }_{\text{Ti}}\) ≈ 4500 kg·m–3 [69]). Since in this position the sinker (\({m}_{\text{S}}\) ≈ 60 g [69]) is coupled with the balance, the total load on the balance is again approximately 82 g, as in the tare position. In this way, errors caused by the non-linearity of the analytical balance used are significantly reduced. As both compensation weights have the same volume, the buoyancy effect of the ambient air on the weights is compensated for.
3.4.3 Compact Version of the Single-Sinker Densimeter
To further simplify the single-sinker densimeter, a compact version was developed by Docter et al. [75, 76] in the 1990s, which was briefly described by Wagner and Kleinrahm [24] and whose basic design is illustrated in Fig. 11 of reference [24]. In this version, the measuring cell and the coupling housing of the MSC have been unified, resulting in a very compact design that requires smaller amounts of the fluid sample. The cylindrical sinker, with a hole through its central axis, is made of, e.g., single-crystal silicon (\({\rho }_{\text{S}}\) ≈ 2330 kg·m–3); its volume is approximately 20 cm3. On the basis of this compact version of the single-sinker densimeter, an apparatus for the combined measurement of the viscosity and the density of fluids was developed by Docter et al. [75, 76]. Further, a special single-sinker densimeter based on such a compact version capable of achieving cryogenic temperatures was developed by Richter et al. [118]; its design is illustrated in Figs. 1 and 2 in [118]. Compact versions of single-sinker densimeters have been used or are still in use in many institutes, as listed in Table 1.
3.5 Other Densimeters and Applications
Beyond the two-sinker densimeters and single-sinker densimeters, researchers have also modified existing commercial systems or developed new systems for other applications in thermodynamics, e.g., a tandem-sinker densimeter [22, 23, 125, 126], a four-sinker densimeter [127, 128], and a magnetic suspension mass comparator [135,136,137].
3.5.1 Tandem-Sinker Densimeter
In order to carry out a comprehensive uncertainty analysis, especially regarding the FTE, of an MSC in a single-sinker densimeter or a commercial gravimetric sorption analyzer (based on an MSB with three positions, see Sect. 2.4), Kleinrahm et al. [22] and Yang et al. [125] modified an existing gravimetric sorption analyzer to create a “tandem-sinker densimeter” (as named in their paper). The major modification is the replacement of the sample basket at the bottom position (see Fig. 5) with another solid sinker, which was previously done in a similar way by May et al. [138]. The mechanical mechanism inside the high-pressure cell for sinker lifting and supporting was modified as well, as shown in Fig. 1 of the paper of Kleinrahm et al. [22]. The second sinker could be used for accurate density measurements as well. With such a tandem-sinker densimeter, new insights into the impact of the FTE to density measurements were gained [22]. Subsequently, various solid sinkers with relatively large surface-to-volume ratios and different surface materials (e.g., without any treatments, galvanically gold plated and sandblasted with 250 μm particles, see Fig. 1 of [23]) were used in the bottom position of the tandem-sinker densimeter. This allowed for the quantification of the fluid adsorption on the sinker surface and its impact on accurate density measurements near the dew point of fluids [23, 139].
Adopting a similar idea of developing a tandem-sinker densimeter, a dual-basket sorption analyzer was developed by replacing the sinker at the top position of a gravimetric sorption analyzer (see Fig. 5) with another basket. The needed density values can be calculated with an equation of state (EoS) using the measured temperature and pressure. This idea was first adopted by Pouya 2023 [140] based on their commercial gravimetric sorption analyzer [141, 142], which significantly improved the measurement efficiency since two adsorbent samples could be studied at the same time rather than just one.
3.5.2 Four-Sinker Densimeter
As revealed by Richter and Kleinrahm [143], there is the need for a more accurate measurement technique for vapor-phase density measurements that accounts for the impact of adsorption phenomena on solid surfaces near the dew point. The state-of-the-art two-sinker densimeter is not able to account for such impacts fully, especially when the composition of a mixture changes due to adsorption. Even density measurements of pure fluids are affected by high adsorption in the vicinity of the dew point. Against this background, a novel four-sinker densimeter was designed and fabricated by Moritz et al. [127, 128]. The system is based on the well-established two-sinker density measurement principle with the additional capability of quantifying adsorption effects. It combines the traditional two-sinker technique, by means of two ‘density sinkers’ for accurate density measurements, with two additional ‘sorption sinkers’ (see Fig. 9). The system was designed for measurements over the temperature range from (190 to 470) K at pressures up to 15 MPa. A major advancement of the four-sinker densimeter is that all four sinkers can be weighed independently in the same coupling position, which largely cancels the FTE.
Cut-away of the four-sinker densimeter’s measuring cell and the view of the four sinkers in center of the measuring cell; two top sinkers for density measurement and two bottom sinkers for adsorption characterization. (Figure taken with minor modifications from the dissertation of Moritz [128].)
The two ‘density sinkers’ have similar geometrical surfaces and are both gold plated in order to have comparable adsorption loads. Both sinkers have the same mass but different volumes as one is made of silicon and the other of stainless steel. The large volume difference increases the resolution of density measurements even for low densities. The two ‘sorption sinkers’ have much larger surface areas than the ‘density sinkers,’ which allows for the detection of gas adsorption onto the sinker surface with relatively low uncertainty. Both ‘sorption sinkers’ are made of stainless steel and are identical in construction except for different surface finishes: one is gold plated. The two surface finishes of the ‘sorption sinkers’ resemble the internal surfaces of the instrument (gold-plated measuring cell, and, e.g., stainless steel gas distribution line). Hence, accurate dew-point densities can be determined based on the simultaneous measurement and correction for adsorption phenomena.
3.5.3 MSB for Mass Metrology
The 2019 redefinition of the International System of Units (SI) presented a challenge for the dissemination of the unit of mass. Prior to 2019, the kilogram was defined as the mass of a cylinder of platinum-iridium, known as the International Prototype of the Kilogram (IPK); in other words, the kilogram was the mass of a physical artifact. The IPK would be used to calibrate national standard kilograms, which, in turn, were used to calibrate working standards. All these comparison weighings were carried out in normal air. The new definition of the kilogram is based on the Planck constant, which can be measured extremely precisely with a Kibble balance weighing a standard kilogram. Once the highest-achievable precision was achieved, the Planck constant’s value was fixed and the definition of kilogram inverted, removing its dependence on the IPK. With the new definition of kilogram, a Kibble balance determines the absolute mass of a standard in vacuum.
However, practical mass metrology still relies on standard masses (i.e., physical artifacts) with weighings carried out in air. To maintain their stability, the Kibble-balance-calibrated masses are always held under vacuum, while the conventional masses are always held in air. The dissemination of the kilogram thus requires a comparison of a standard mass in vacuum with a working standard in air. To address this challenge, Stambaugh and coworkers at NIST developed a “magnetic suspension mass comparator” (MSMC) [135,136,137], as schematically illustrated in Fig. 1 in [137]. A commercial mass comparator and the Kibble-balance-calibrated mass are located in an upper chamber kept in vacuum. A weighing pan, the carriage, and the mass to be measured are magnetically suspended in a lower, air-filled chamber through the vacuum-to-air boundary flange using an MSC.
This design enables direct comparisons to be made between masses in vacuum in the upper chamber with masses in air in the lower chamber. The technology required to achieve a comparison of objects to a precision of 1 part in 108 (i.e., a precision of 0.01 mg for 1 kg objects) was quite sophisticated. The complexity of the MSMC precludes its general application to density or adsorption measurements, but it does illustrate the potential of MSB technology.
3.6 Applications
Nowadays, there are many software packages (such as REFPROP 10.0 [144], TREND 5.0 [145], and CoolProp 6.4.1 [146]) for accurate thermophysical property calculations of industrially important pure fluids and fluid mixtures. These packages, playing important roles in both industrial applications and scientific research, are based on reference EoS for thermophysical properties of each pure fluid and their binary pairs. Among these reference equations, most are multi-parameter Helmholtz EoS, which were fitted using comprehensive high-accuracy experimental data. The density measurements using MSB-based densimeters were generally evaluated as having the highest quality and are almost always given high weight in developing such fundamental Helmholtz EoS; Table 2 gives examples. Please note, not all experimental data of pure fluids listed in Table 1 were used for reference Helmholtz EoS fitting because either the current reference EoS (e.g., for propylene [147], toluene [148], R-32 [149], n-heptane [150], n-nonane [148]) was developed before the measurements were available, or the reference EoS is yet to be developed (e.g., for 2,4-dichlorotoluene and bromobenzene) or published (e.g., neon [144]).
In recent years, the MSB-based densimeters have been used more for measurements of fluid mixtures than pure fluids. The measured fluids are mainly: natural gas [48, 61, 87,88,89,90,91,92, 143] and mixtures of natural gas components [49, 64, 98, 102,103,104, 117,118,119,120,121, 124] for the gas and oil industry, CO2 mixtures [25, 50,51,52,53,54, 100,101,102, 116, 117] in advancement of a low-carbon society, refrigerant blends [106,107,108,109, 111,112,113,114,115] for environmentally friendly next-generation refrigeration, and H2 mixtures [49, 52, 53] and biomethane-like mixture [122] for renewable energy. Many of these measurements were also used for fitting the reference EoS of fluid mixtures, e.g., data from references [61, 90, 91, 98] for the GERG-2008 EOS [167], data from references [99,100,101,102] for the EOS–CG [168], data from references [49, 118, 119, 124] for the EOS–LNG [169].
In addition to providing highly accurate fluid density (and some viscosity) data largely for the fields of energy and the environment, the MSB-based densimeters were also used for fundamental research. The two-sinker densimeter at NIST and the tandem-sinker densimeter at TUC (originally at RUB) were both used for the highly accurate quantification of adsorption phenomena on solid surfaces near the dew point [23, 126, 131], which are less likely to be measured by other measurement techniques. The four-sinker densimeter, currently under development at TUC, is aimed at highly accurate density measurements of fluid mixtures near and on the dew line, where adsorption is so significant that ordinary techniques or typical MSB-based densimeters fail to eliminate its effect on density measurements (see Sect. 3.5.2).
4 Application in Sorption Science
4.1 List of MSB-based Sorption Analyzers
Although the MSB was originally developed for accurate fluid density measurements, it turns out that the major application of the technique lies in the field of sorption science. This was attributed to the great success of the product of Rubotherm (now TA Instruments): Gravimetric Sorption Analyzer—IsoSORP. For many years, IsoSORP was the only commercially available system that measured adsorption isotherms of gases on porous materials up to very high pressure (e.g., 30 MPa) using a gravimetric technique. A list of institutes with publications using an MSB-based sorption analyzer is given in Table 3, which is based mainly on the results of a search in https://scholar.google.com/ using the keyword “Rubotherm IsoSORP.” Please note, Rubotherm GmbH was founded in 1990 and most of its “MSB-based sorption analyzers” delivered in the 1990s were MSBs with two positions. The Rubotherm IsoSORP was first introduced in the early 2000s and it is based on an MSB with three positions. The earlier papers (before 2000) were not found under the keyword “Rubotherm IsoSORP” and were very few. For each institute, to reduce the size of this review paper, only the oldest and most recent references are listed; all the literature found in this review are listed in Table S1 in the supporting information (SI). The applications in Table 3 are based on the title and keywords of the referenced literature.
The number of peer-reviewed publications and the number of institutes with their first published paper based on an MSB-based sorption analyzer during each 3-year period are statistically illustrated in Fig. 10a and b, respectively. Please note, Fig. 10 is based on Table S1 in the SI (Table 3 in the paper is a reduced version), where it cannot be guarantee that all institutes and publications have been listed. It is clear that the number of publications is increasing almost exponentially. Also, the present review was completed in March 2023, and there will be more publications in 2023. The number of new systems, represented by their first publication, is also increasing exponentially. However, as shown in Fig. 10b, there is a decrease from the period 2016–2019 to 2020–2023. The main reasons could be (1) the present review was completed in March 2023 and (2) the COVID-19 pandemic hindered the development of new systems.
(a) Number of peer-reviewed publications and (b) number of institutes with their first published paper based on an MSB-based sorption analyzer. (c) Number of institutes that have published papers using an MSB-based sorption analyzer, by country. The ‘others’ in (c) refers to 12 countries (Denmark, Spain, UAE, Brazil, Belgium, France, Switzerland, Poland, Qatar, Netherlands, Sweden, Canada) each having one instrument
According to Table 3, there are more than 80 institutes in more than 20 countries that have publications using an MSB-based sorption analyzer. The number of institutes categorized by countries is shown in Fig. 10c. There are 31 institutes owning an MSB-based sorption analyzer in China, followed by Germany (where Rubotherm was founded) and the United States, both with 11 institutes each. The other 20 countries, see Fig. 10c, own 31 sorption analyzers. Most of the MSB-based sorption analyzers in China were setup over the last 10 years.
4.2 Pure Fluid Adsorption
The first MSB-based sorption analyzer was developed by Dreisbach and Lösch [21]. As mentioned in Sect. 2.4, this system was especially designed for simultaneous measurements of gas adsorption on the surface of porous materials and the gas density. This system has been used for adsorption measurements of pure CH4, H2S, and their binary mixtures on a zeolite sample [21] as well as pure CH4, N2, CO2, and their ternary mixtures on activated carbon [311].
To describe the measurement principle of pure-gas adsorption using an MSB-based sorption analyzer as illustrated in Fig. 5, it is necessary to describe the differences between the three concepts of gas adsorption: net, excess, and absolute adsorption. The details are given in [312,313,314]; here, only a brief explanation is given based on Fig. 1 of Yang et al. [314]. At the same temperature and pressure, in a closed pressure vessel with a fixed volume, the net adsorbed mass, mnet, could be considered as the difference between the total masses of the gas (including adsorbed gas) in the vessel with and without the porous material. The net adsorbed mass, mnet, is related to the excess adsorption mass, mnet, as
where VP is the skeletal volume of the porous adsorbent sample. The absolute adsorbed mass, mabs, is the mass of the adsorbate (adsorbed gas), and it is related to mexe as
where Vsorb and ρsorb are the volume and density of the adsorbate, respectively, with Vsorb · ρsorb = mabs. In application, mnet is an important value to assess an adsorbent’s capability for a gas storage application, mexe is the direct (compared to mnet and mabs) measurement result of a sorption analyzer based on a volumetric technique, and mabs is directly modeled by most of the adsorption models (e.g., Langmuir model, Toth model [315]).
In an MSB-based sorption analyzer, two weighings of the adsorbent sample with its lifting mechanism (sample basket and lift rods see Fig. 5, which are typically made of a stable material, relatively lightweight, and have minimal adsorption characteristics of their own) are carried out with the measuring cell being evacuated, mSL, and filled with fluid, \({m}_{\text{SL}}^{*}\), respectively. According to the Archimedes’ buoyancy principle:
where VL is the volume of the lifting mechanism. Combining Eqs. 5–7 yields
According to Eq. 8, the net adsorbed mass, mnet, is a direct measurement (compared to mexe and mabs) with a gravimetric technique.
The volume of the lifting mechanism, VL, can be determined by conducting a typical isothermal measurement using pure nitrogen without adsorbent sample in the basket, and VL is the slope of the data set (ρfluid, \({{m}_{\text{L}}^{*}-m}_{\text{L}}\)), where mL and \({m}_{\text{L}}^{*}\) are the weighings of the lifting mechanism with the measuring cell being evacuated and filled with nitrogen, respectively. The skeletal volume of the adsorbent sample, VP, can be determined by a similar method but with the sample in the basket and using pure helium instead of pure nitrogen assuming helium is a non-adsorbing gas on any adsorbent. There is no experimental technique for measuring the volume, Vsorb, or density, ρsorb, of the adsorbate accurately, nor is there a reliable model to predict them. The values of Vsorb and ρsorb can only be estimated, e.g., by the Ono–Kondo model [316].
4.3 Gas Mixture Adsorption
The measurement of gas mixture adsorption equilibria remains one of the most challenging fronts in the adsorption field. The first MSB-based sorption analyzer developed by Dreisbach and Lösch [21] provided such data, but the excess adsorption and absolute adsorption are not distinguished in their data analysis and there is no mechanism to achieve thorough mixing of the gas mixture. A comprehensive review given by Shade et al. [317] in 2022 identified 18 experimental techniques for gas mixture adsorption equilibria. Those based on an MSB-based sorption analyzer are always very complicated, combining either gas composition analysis equipment, such as a gas chromatograph, or a volumetric system [318].
Based on the pioneering work of Dreisbach and Lösch [21], Yang et al. [319] made minor modifications to a commercial gravimetric sorption analyzer and proposed a new data analysis method for the measurement of binary gas adsorption equilibria. The modified system did not rely on gas composition analysis equipment, thus, the accuracy was not limited by such equipment, and it was also less complicated than the volumetric–densimetric method [318]. Compared to pure-gas adsorption measurements, for the binary gas adsorption equilibria, only one extra value, i.e., the internal volume of the high-pressure measuring cell, Vcell, (or more specifically, the internal volume of the cell with the sinker and the lifting mechanism inside the cell and without adsorbent sample), needs to be measured. The measurement principles are described in detail in Yang et al. [319], and the system is illustrated in Fig. 1 of [319]; only the key information is given here.
Similar to pure-gas adsorption measurements, temperature, Tgas, pressure, pgas, density, ρgas, of the gas mixture inside the measuring cell, and the net adsorbed mass, mnet, of the gas mixture on the porous material can be acquired. With Tgas, pgas, and ρgas known, the mass fractions, ym1 and ym2, and the mole fractions, y1 and y2, of each component in the gas phase can be calculated using an EoS (e.g., GERG-2008 EoS [167]). Please note, y1 and y2 are values at adsorption equilibrium, which are different from those of the injected gas y1,o and y2,o (e.g., reported by the gas supplier).
According to the definition of the net adsorbed mass, mnet, as discussed in Sect. 4.2.1, the total mass of the gas injected into the measuring cell, minj, can be calculated with
and the absolute adsorbed mass, mabs, can be calculated with
The value of ρsorp for mixtures can be estimated as the reciprocal of the average molar volume of the pure components. The absolute adsorbed mass of each component, m1 and m2, on the adsorbent sample can then be determined by solving the following mass balance equations:
The results are as follows:
With m1 and m2 determined, the mass fractions, xm1 and xm2, and mole fractions, x1 and x2, of each component in the adsorbed phases can be easily calculated.
4.4 Applications
4.4.1 Application Category
Based on Table 3, the number of institutes categorized by applications is shown in Fig. 11. Here, a sorption analyzer in an institute might have multiple applications. We are not experts in each research field, thus, there is no guarantee that the respective assigned application category is the best one. However, qualitative results can still be obtained. Sorted by the number of institutes, sorption analyzers were mainly used in the following applications: carbon capture and storage, shale gas exploration, porous material characterizations (mainly metal–organic framework), hydrogen storage/fuel cells, ionic liquid characterization, membrane studies, other gas sorption studies (mainly methane), and others.
The applications mainly lie in capture and removal of CO2 for environmental protection, as well as adsorption and storage of CH4 and H2 in the energy sector. Carbon capture and storage (CCS) is the process of capturing carbon dioxide emissions from power plants and other industrial sources and storing them underground or reusing them for industrial purposes; CCS is an important technique to achieve a low-carbon society. Research into membranes or adsorbent materials for CO2 separation and storage is one of the greatest applications of MSB-based sorption analyzers. Shale gas, with CH4 as the main component, is an important energy source extracted from shale formations and is an unconventional energy resource, which is cleaner compared to coal and oil. Most of the research over the past 6 years from China has focused on shale gas exploration and the relative mechanism studies. Hydrogen is non-polluting and produces no harmful emissions throughout the cycle if the hydrogen is produced from renewable sources. Hydrogen storage in an affordable way is a bottleneck toward large-scale adoption of hydrogen energy applications. Lots of research using MSB-based sorption analyzers is intended to identify suitable porous materials, mainly metal–organic frameworks (MOFs), for hydrogen storage.
Recent research has also focused on the development of new materials for gas adsorption and separation processes, including zeolites, MOFs, ionic liquids (ILs), and activated carbon. In recent years, MOFs have gained considerable attention due to their large surface area and tunable pore size. Finally, MSB-based sorption analyzers are also used in other fields, such as the development of fuel cell membranes and the production of microcellular biodegradable polymeric foams. Some projects are also focused on the adsorption of gases in foodstuffs, such as shrimp and fish mince, indicating the diverse applications of MSB adsorption analyzers.
4.4.2 Scientific Impacts
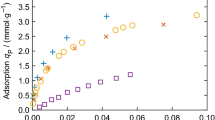
It has been commonly recognized that the results of adsorption measurements of the same gas on the same type of porous material performed by different research groups show large deviations. An example is illustrated in Fig. 1 of [125], where the relative deviations of the measured CO2 adsorption on zeolite 13X (a commercially available standardized porous material) at the same temperature and pressure conditions of four different groups [320,321,322,323] are higher than 100%. Another example is given in Fig. 12, where hydrogen adsorption on IRMOF-1 (also called MOF-5, which is the most studied material for hydrogen storage) at T = 298 K at the highest pressure measured by different authors [324,325,326,327,328,329,330,331,332,333,334,335,336,337,338,339,340,341,342,343,344,345,346,347,348,349,350] are plotted. It is clear that there are significant deviations among different measurements, and it is hard to judge which measurements can be trusted.
Hydrogen adsorption on IRMOF-1 (MOF-5) at 298 K measured by different authors [324,325,326,327,328,329,330,331,332,333,334,335,336,337,338,339,340,341,342,343,344,345,346,347,348,349,350] with only the values at the highest pressure shown. Data were obtained from the NIST isotherm database (ISODB) [351]
Against this background, there is increasing research aimed at the standardization of gas–solid adsorption measurements. The most typical research is that carried out by Nguyen et al. [352, 353]. In their work, twenty laboratories participated in international inter-laboratory studies led by NIST on the measurements of high-pressure gas adsorption on NIST reference materials. Reference equations of the adsorption isotherms for the studied systems were developed, which is a big step toward the standardization of sorption experiments. MSB-based sorption analyzers play a key role in such studies. For example, for the CO2 adsorption measurements on NIST Reference Material RM 8852 (ZSM-5) [353], 11 gravimetric systems and 18 volumetric systems were involved. Measurement results from all gravimetric systems, compared to only 10 of 18 volumetric systems, were utilized for reference equation development. According to Table 3 and [353], one can infer that most of the 11 gravimetric systems are MSB-based sorption analyzers.
In addition to producing highly accurate experimental data for the standardization of sorption measurements, MSB-based sorption analyzers are commonly used to provide reference data for the development of novel sorption measurement systems based on techniques such as Raman spectroscopy [354] and nuclear magnetic resonance [283, 285, 355]. Furthermore, many of the MSB-based sorption analyzers are used as they are, while some institutes modify their system for various purposes. For example, a tandem-sinker densimeter (see Sect. 3.5.1) was built by replacing the basket with another sinker for the quantification of gas adsorption on quasi-non-porous materials [23, 126, 139], and comprehensive systems were built combining an MSB-based sorption analyzer with other measurement techniques for binary gas adsorption measurements [318].
5 Measurement Uncertainty
MSBs are capable of very low uncertainties, but these are not automatically obtained—careful attention to several aspects of the experiment is required. The uncertainties actually obtained vary widely among different MSB instruments, and it is not possible to state an uncertainty for this entire class of instruments. Rather, we summarize some of the important effects that impact measurement uncertainty.
5.1 Weighings
Weighings of a sinker or adsorbent sample are at the heart of all MSB measurements, and uncertainties in these weighings arise from the used balance, the MSC itself, and the stability of conditions in the laboratory and measuring cell. Modern analytical balances are capable of extraordinary precision. A resolution of 1 μg for loads of 100 g (1 part in 108) is available with commercially available “mass comparator” balances, and resolutions of 10 μg for loads of 200 g (5 parts in 108) are common for high-quality analytical balances. The reproducibility of a weighing is typically 3 to 5 times the resolution, and the linearity (i.e., the uncertainty over the entire weighing range) is typically 10 to 15 times the resolution. The linearity effect can be greatly reduced by employing compensation masses (as discussed in Sect. 3.4.2) so that the total load on the balance is similar for the different weighings, and this would bring the balance uncertainty close to the reproducibility. It must be recognized that balances will drift with changes in ambient conditions, including changes in barometric pressure (i.e., air density in the laboratory). This is not a defect of the balance, but a result of the internal structures of the balance being affected by air buoyancy effects. Thus, regular calibration of the balance is essential to achieve its quoted performance; ideally, this would be performed as an integral part of each measurement, by using, for example, the built-in calibration masses of the balance or the compensation masses (see Sect. 3.4.2).
A well-tuned MSB (i.e., one that is stable to the level of the balance precision) is equally important to maintain the precision of the balance and minimize weighing errors. While the tuning of the MSB is a job for the manufacturer (or exceptionally experienced users), the alignment of the balance/coupling/sinker system is clearly the responsibility of the user. If any part of the coupling or sinker (or adsorbent sample) contacts the wall of the coupling housing or measuring cell, stable weighings will be impossible. The weighing uncertainty is also affected by the stability of the conditions inside the measuring cell. Any changes in the density of the fluid (whether by changes in temperature or pressure in a density experiment or resulting from adsorption) will, of course, affect the weighings. Instability of the temperature can give rise to convection currents, which can exert forces on the sinkers.
With a well-tuned and aligned MSB and stable conditions inside the measuring cell, the reproducibility of weighings in a density experiment can approach the reproducibility of the balance itself. (A sorption experiment, by its nature, involves a changing mass of the object and is likely to have larger variations.) And considering that the balance is typically under a draft hood and the different objects being weighed are loaded with a sinker-changing mechanism or (in the case of compensation masses) robotic arms, the balance may even give more stable results than a manual weighing involving the opening of doors and the thermal influence of a human operator. As an example, a weighing precision of 10 μg for a density sinker with a volume of 10 cm3 yields a density precision of 0.001 kg·m–3, which is 0.0001% (1 ppm) for a liquid-like density or 0.08% for the density of ambient air. But the overall uncertainty will be substantially higher due to effects discussed in the following sections.
5.2 Force Transmission Error
A properly designed MSC very efficiently transmits the forces acting on the sinker to the balance, but it is slightly influenced by nearby magnetic materials, external magnetic fields, and the fluid being measured. These give rise to a “force transmission error” (FTE), which was discussed by Brachthäuser et al. [68], Klimeck et al. [70], and McLinden et al. [356] for densimeters, and by Kleinrahm et al. [22] and Yang et al. [125] for both densimeters and gravimetric sorption analyzers. The FTE of densimeters has also been analyzed for special applications in references [357, 358]. With the appropriate analysis, the force transmission “error” becomes an effect that can be calculated and compensated for rather than a source of significant error in measurements.
The comprehensive FTE analysis carried out by Kleinrahm et al. [22] using a tandem-sinker densimeter concluded that, for density measurements, the FTE must be accounted for to realize the full accuracy of MSB, while for adsorption measurements on porous material, the FTE is negligibly small compared to other uncertainty sources. Therefore, here, we only provide a brief summary of the FTE analysis for two-sinker densimeters and single-sinker densimeters, based on McLinden et al. [356].
The analysis for a two-sinker densimeter starts with writing out the forces acting on the balance for each of the weighings; these include not only the sinker, but also the permanent and electromagnets of the MSB and air buoyancy forces. For the weighing of sinker 1
where α is a balance calibration factor, ϕ is the “coupling factor” due to FTE, Wzero is the balance reading with nothing on the balance pan, and the subscripts p-mag and e-mag refer to the permanent magnet (in the fluid and including the lifting device) and to the electromagnet (in air), respectively. The key assumptions implicit in Eq. 15 are that (1) the force transmitted to the balance by the MSC is proportional to the suspended load and this is characterized by the coupling factor ϕ, (2) all quantities are constant over the time needed for a complete density determination, and (3) the balance reading is linear with the applied load. The electromagnet and permanent magnet (plus lifting device) are always weighed, and the Wzero is the same for each weighing, so that these can be lumped together as
Similar equations can be written for the weighing of the second sinker and also for the separate weighings of the two compensation masses directly on the balance pan (with the coupling in zero position). The result is a system of four equations with the four unknowns ρfluid, α, β, and the coupling factor ϕ. A coupling factor ϕ = 1 corresponds to a “perfect” coupling. The balance factor α accounts for the fact that a balance is a force transducer, but displays mass units. Furthermore, by convention, a balance will read “1.000 g” when a 1 g standard mass, with a density of 8000 kg·m–3, is placed on the pan, even though it will experience an air buoyancy force of approximately 0.15 mg per gram.
McLinden et al. [356] go on to demonstrate that the ϕ can be divided into apparatus and fluid-specific contributions. ϕ0 is the value of ϕ in vacuum and is the apparatus contribution; it is obtained by weighing the sinkers in an evacuated measuring cell. The fluid-specific effect is proportional to the fluid density and the specific magnetic susceptibility of the fluid χs, with a proportionality constant ερ:
The coupling factor ϕ is shown in Fig. 13 as a function of density for measurements on propane (a diamagnetic fluid with χs = –1.10 × 10–8 m3·kg–1) and air (a paramagnetic mixture with χs varying with temperature from 35.74 × 10–8 m3·kg–1 at T = 250 K to 19.12 × 10–8 m3·kg–1 at T = 460 K). Note that these results are for a particular densimeter (the one at NIST in Table 1) and illustrate only the general trends and the magnitude of the effect.
Coupling factor as a function of density for measurements on air (with T = 250 K to 460 K) and propane; figure adapted from McLinden et al. [356]
A similar analysis can be carried out for a single-sinker densimeter, except that there are only two or three equations, but still four unknowns. The balance calibration can be integrated with the sinker weighings (using compensation masses) or carried out separately to obtain α. Weighings in vacuum yield the ϕ0, but the fluid-specific effect (as quantified by the parameter ερ) must be obtained by a separate determination. There are two methods for obtaining ερ. The first involves measurements with a fluid at the same (p, ρ, T) at different times using two different sinkers; thus, creating a sort of “sequential” two-sinker densimeter that allows an analysis by the above equations. A simpler, but less accurate, approach requires measurements on a fluid with a well-characterized magnetic susceptibility, such as oxygen (or a well-known oxygen–nitrogen mixture), and comparison with an accurate EoS.
The magnitude of ϕ is typically 1 ± 0.000 020. Ignoring the FTE corrections in a two-sinker densimeter will result in a relative (percentage) error that is small (typically less than a few 10’s of ppm), except for strongly paramagnetic fluids. For a single-sinker densimeter, however, the force transmission errors can be substantially larger, and ignoring the FTE introduces an absolute error that is proportional to (ρsinker – ρfluid). This is most pronounced at low densities. The apparatus portion of the FTE can be determined with a simple experiment in vacuum, and this correction should always be applied. Taking the example of a 60 g sinker of silicon with a volume Vs = 25.76 cm3, a force transmission error of 1.2 mg (i.e., 60 g · (1 – 1.000020)) would result in an error in density of 0.047 kg·m–3. For a liquid density of 1000 kg·m–3, this would be 0.0047%, but for a gas at 20 kg·m–3 the error would be 0.14%.
The above analysis of FTE is largely empirical. Kuramoto et al. [83] and also Kano et al. [359] presented a physical model for the FTE, but they are complex and require detailed knowledge of the magnetic properties of both the apparatus and fluid, which may not be available, and would be applicable only to a specific instrument, in any case.
A recent analysis of the FTE by Kleinrahm et al. [22], which focuses on single-sinker densimeters, describes the correction of the FTE in an alternative way:
where ρfluid is the density of the fluid, ρexp,uncorr is the measured density without correction of the FTE, εvac is the apparatus contribution of the FTE (which is usually less than ± 0.000 020, but it depends on temperature), and εfse is the FTE contribution of the fluid being measured (fluid-specific effect). The fluid-specific effect can easily be calculated by
where ερ is a proportionality constant (typical value 0.000 053), χs is the specific magnetic susceptibility of the measured fluid, χs0 = 10–8 m3·kg–1 is a reducing constant, ρs is the density of the sinker used (e.g., ρs ≈ 2329 kg·m–3 for single-crystal silicon), and ρ0 = 1000 kg·m–3 is also a reducing constant. By use of Eq. 19, errors due to the fluid-specific effect can be calculated; e.g., for ρfluid/ρ0 = 0.1 and ρs/ρ0 = 2.329, the error is εfse = 0.000 161 for methane (diamagnetic fluid, χs/χs0 = − 1.36), and εfse = 0.003 224 for air (paramagnetic fluid, χs/χs0 ≈ 27.29 at T = 293.15 K). Note, in the case of two-sinker densimeters, the term ρs/ρ0 does not exist in Eq. 19, e.g., ρs/ρ0 = 0.
Since we know that the environment of the MSC (coupling housing and fluid sample) is not completely magnetically neutral, the cause of the FTE can be explained briefly as follows. The increase in the strength of the magnetic field and the increase in the force transmitted from the permanent magnet to the electromagnet are approximately proportional to the lifting height of the permanent magnet in the entire range between the tare position and the measuring position. As a consequence, also the FTE is approximately proportional to the lifting height of the permanent magnet; this applies to both cases, for the evacuated measuring cell and the cell filled with fluid.
For example, the application and effectiveness of this correction method can be seen in the result of the density measurement of a mixture (0.21 oxygen + 0.79 nitrogen, mole fraction). These measurements cover a temperature range from (100 to 298.15) K at pressures up to 8 MPa and have been published in 2021 by von Preetzmann et al. [123]. With respect to strongly paramagnetic fluids, a special study of Lozano-Martin et al. [357] should be mentioned. To determine the fluid-specific effect of oxygen on the single-sinker densimeter used by their group, they measured the density of pure oxygen in the temperature range from (250 to 375) K at pressures up to 6.1 MPa. The results should serve as a basis for future measurements on oxygen-containing gas mixtures.
5.3 Density Uncertainties due to Sinker Effects
The basic working equations of an MSB densimeter, i.e., Eqs. 1 and 2, require the mass and volume of the sinker(s). A determination of the sinker mass can be carried out with the balance used on the densimeter according to standard protocols (e.g., the double-substitution weighing design of Harris and Torres [360]). It is important to note that balances are calibrated with standard masses with a density of approximately 8000 kg·m–3, and a direct reading of the balance display will be correct only for objects of similar density. The standards can be standard masses manually placed on the balance pan or the built-in standards in balances with an automated calibration function. Densimeter sinkers, on the other hand, are typically of significantly lower or higher density, and air buoyancy effects must be taken into account for an accurate mass. This effect amounts to approximately 0.04% for a silicon sinker (ρSi ~ 2330 kg·m–3).
Sinker volumes are equally important in Eqs. 1 and 2; for example, a detailed uncertainty analysis of the NIST two-sinker densimeter [130] revealed that uncertainties in the sinker volumes were the largest source of error, especially at high or low temperatures. Sinker volumes are typically determined with a hydrostatic (weighing-in-water) technique taking the density of the water as a known quantity. This can yield volumes with an uncertainty of 0.01% (or lower), but great care is required to minimize systematic errors due to surface tension, gas bubbles, etc. McLinden and Splett [130] describe a hydrostatic comparator technique based on Bowman and Schnoover [361, 362] where the unknown object (i.e., sinker) is compared to a solid density standard. The density of the fluid need not be known, and this allowed the use of a high-density (ρ ~ 1630 kg·m–3) fluorinated liquid which increased the buoyancy effect on the sinker (and thus the sensitivity of the measurement). This liquid also had a much lower surface tension and larger ability to dissolve gases compared to water, minimizing systematic errors. They used standards of single-crystal silicon with density uncertainties of 0.2 ppm, as determined by Physikalisch-Technische Bundesanstalt (PTB). The result was a volume uncertainty of the sinkers of approximately 10 ppm at t = 20.0 ˚C, p = 0.1 MPa.
The calibration of sinker volumes is typically carried out at 20 ˚C, and the volume as a function of temperature and pressure can be a major source of uncertainty. This is often calculated from materials properties (e.g., Eq. 3); here lies the major advantage of single-crystal silicon, since its thermal expansion is known very accurately (e.g., Richter et al. [118], Appendixes A2 and A3). Fused quartz (quartz glass) is also good, because of its low thermal expansion coefficient. Metal sinkers are more problematic; literature values for the thermal expansion of metals often have large uncertainties, and they can vary significantly depending on the exact alloy and even heat treatment.
Given the above discussion, it might seem like an in-situ calibration of the sinker volume with a known fluid would be easier, as Yang et al. [25] did using pure N2. In this way, other uncertainty sources like sinker weighings, and temperature and pressure measurements are taken into consideration as well. The uncertainty of the densimeter can be relatively easily estimated according to the calibration results; however, the ‘calibrated’ sinker volume might be distorted from the true value due to other uncertainty sources, mainly the pressure measurement. Nevertheless, for moderate accuracy requirements, several liquids can be used as density reference fluids, e.g., n-heptane, n-nonane, 2,4-dichlorotoluene, and bromobenzene as measured by Schilling et al. [73], and cyclohexane, toluene, and ethanol as measured by Sommer et al. [74]. These density measurements cover a temperature range from (233.15 to 473.15) K at pressures up to 30 MPa. The uncertainty is given as 0.02% or 0.015% in most cases, and the uncertainty of the fitted correlation equations is given as 0.02% in most cases. The seven mentioned fluids can serve as reference fluids for the calibration of the pressure and temperature dependence of the sinker volume in a large temperature and pressure range. However, the volume of the sinker at reference conditions, e. g., T = 293.15 K, p = 0.1 MPa, must be calibrated with another reference liquid.
5.4 Adsorption Measurements
With great efforts spent on calibrations and improvements of a densimeter, very low density-measurement uncertainty could be achieved, e.g., generally on the order of 0.02% to 0.08% as listed in Table 1. However, for adsorption measurements, as studied by Yang et al. [125], the major uncertainty sources for the adsorption capacity (q) is the purity and then the skeletal volume of the adsorbent sample. If absolute adsorption capacity, qabs, is the target, the estimation of the density of the adsorbate (ρsorp) is also a significant source of uncertainty. The impurities of a porous material include not only the different solid materials and defects of the structure attributed to the sample synthesis, but also the remaining adsorbate due to the incomplete activation after the previous measurement. Quantifying the impurity is difficult; the 2% used in [125] is simply an empirical estimation. A 10% uncertainty in the estimation of ρsorp could yield approximately 1% uncertainty in adsorbed mass (and capacity), see Table 4 in [125]. Without considering the above two major uncertainty sources, the remaining effects (temperature, pressure, calculated or measured density, mass, and volume of the adsorbent sample and lifting mechanism) combine to contribute approximately 0.2% [125]. Among the remaining uncertainty sources, mass and volume of the porous material are the dominant factors.
According to binary gas adsorption equilibria (8), to estimate the uncertainty of the net adsorption mass, mnet, the uncertainty in mass weighings of the activated sample and lifting mechanism, fluid density (or density calculation using an EoS with measured temperature and pressure), and volume of the lifting mechanism need to be determined. For uncertainty estimation of the excess adsorption mass, mexe, an additional contributor, the skeletal volume of the adsorbent sample, VP, needs to be taken into consideration, see Eq. 5; and for absolute adsorbed mass, mabs, an additional factor is the density of the adsorbate, ρsorb, see Eq. 6. In this context, mabs has the highest uncertainty, followed in sequence by mexe and mnet.
6 Challenges and Outlook
6.1 Continued Availability of MSB Systems
Continued development of innovative instruments built up around MSB is threatened by the proprietary nature of MSB technology. Many of the innovations in experimental thermodynamics and sorption science described in this review were developed using custom systems by the founders of Rubotherm GmbH. But, as Rubotherm evolved from a university spin-off (with its attendant receptiveness to explore new research areas) to being a subsidiary of a mainstream instrumentation company (TA Instruments), its focus has shifted to standard products, and custom systems are no longer readily available. A potential successor, Rubolab, was founded in 2014, but was soon sold to JWGB, Beijing, China in 2022.
The expertise to develop, for example, systems capable of magnetically suspending a 60 g load with microgram precision (as is done in the NIST two-sinker densimeter [55]) has apparently disappeared.
While there are recent examples of research groups building up, from scratch, their own MSB systems [67, 83, 135, 363], this requires specialized expertise beyond that of the typical thermodynamics or adsorption research group. There is a need to make public the technology behind MSB before knowledge is further lost. An “open source” design based either on traditional technology or based on direct digital control, which could be built up by a competent electronics technician, would be highly desirable and would enable continued innovations employing MSB technology.
6.2 Outlook for MSB-Based Techniques
According to Fig. 10, there is no doubt that the number of MSB-based sorption analyzers, which are well commercialized, will keep growing. However, there have been no new single-sinker densimeters developed since 2016 and no new two-sinker densimeters since 2012 (see Table 1). In addition to the limitations of availability discussed in Sect. 6.1, there are reduced demands for highly accurate pure-gas density data from industry. As for gas mixtures, experimentalists have long realized that the major uncertainty source is the composition of the mixtures rather than the density measurement techniques. Therefore, less accurate but simpler techniques, such as vibrating tube densimeters, become more attractive for liquid density measurements. Nonetheless, vibrating tube densimeters are hardly suitable for measuring the density of gases because of the large measurement uncertainty at low gas densities. Moreover, other techniques such as torsional resonators must be further performance tested by the scientific community to establish reliable uncertainty statements.
However, the overall need for accurate MSB-based densimetry remains. Very few industrial applications require high-accuracy density data, for its own sake. (The major exception would be the flow metering of chemicals of very high economic value, such as natural gas and other fuels.) Density data have always been required for the development of accurate EoS and the other thermodynamic properties (heat capacity, enthalpy, etc.) needed in process calculations that can be calculated from an EoS. Here, the first and second derivatives of density are required, and these require data of higher accuracy and over wider ranges of temperature and pressure than would be required by the industrial process itself.
Furthermore, while molecular simulation cannot currently provide reliable values of liquid-phase properties, it continues to improve, and as it does so, the need for lower-accuracy data will decrease (just simulate, rather than measure). The need for high-accuracy data, such as those that can be provided by MSB-based densimeters, will still be required to verify simulations. Further advancements in the state-of-the-art of EoS also require high-accuracy data for reference fluids; such advancements would enable reliable EoS to be developed for fluids with lesser data sets.
Scientific applications for MSB-based densimetry remain, most likely as a reference instrument for density measurement in a country’s national metrology institute, such as those of NIST in the USA and the National Metrology Institute of Japan. As far as we know, some National Metrology Institutes (e.g., of China, South Korea and Italy) have shown interest in setting up MSB-based densimetry in their laboratories.
Regarding the future development of the MSB-based densimetry, a potential path could be the simplification of the whole system while avoiding a significant accuracy loss. A complementary path is to extend its capability and, thus, complexity to measurements at cryogenic temperatures. The first step has been done by Richter et al. [118] whose system is capable of measurements down to a temperature of 90 K using liquid nitrogen for cooling. A further step could be the use of liquid helium for cryogenic cooling since it is likely that cryocoolers impact the proper function of the MSC, e.g., due to subtle vibrations or magnetism. This would clearly extend the capability of such techniques down to liquid hydrogen temperature, i.e., T = 20 K. However, in case the MSC is cooled to such low temperatures, challenging issues might arise, e.g., the used cell material becoming magnetic and the permanent magnet becoming extremely strong. Both issues would significantly increase the FTE and thus the measurement uncertainty or might even cause the MSC function to fail. To avoid this problem, the MSC would have to be placed, e.g., at ambient temperature while the measuring cell is at cryogenic temperature. However, when measurements on mixtures are the goal, this would lead to the problem of fractionation described in detail by Richter et al. [118], so that only measurements on pure cryogenic fluids would be possible.
Moreover, a step toward advancing the vapor–liquid equilibrium modeling of fluid mixtures is the provision of accurate dew-point density measurements. There is a need for a more accurate measurement technique for vapor-phase density measurements that accounts for the impact of sorption phenomena on solid surfaces near the dew point. The state-of-the-art two-sinker densimeter is not able to fully account for such impacts, especially when the composition of the gas mixture under study changes due to sorption effects. As mentioned in Sect. 3.5.2, the on-going development of the four-sinker densimeter aims at tackling this challenge.
Finally, extending the capability of an MSB-based sorption analyzer for gas sorption measurements down to liquid nitrogen temperature (77 K) would be of great scientific and industrial interest. Most current sorption measurements at high pressures (e.g., up to 10 MPa) and low temperatures (e.g., 77 K to 273 K) were carried out with a volumetric technique which has much higher uncertainties than a gravimetric one. The resulting data are unreliable and have hindered the development of low-temperature processes based on gas adsorption. A cryogenic MSB-based sorption analyzer could yield much more accurate and reliable data, and thus become indispensable to overcome this bottleneck for the development of those processes. For example, if a porous material could be found for larger volume-density and mass-density of hydrogen storage at T = 77 K than compressed hydrogen at p = 70 MPa, the technique based on this could become widely used in a future hydrogen economy.
Data Availability
All data required to understand the present work are provided in the supporting information file.
Notes
Commercial materials and sources are identified only for documentary purposes. In no case does such identifications imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the products identified are necessarily the best available for the purpose.
References
A.B. Lamb, R.E. Lee, J. Am. Chem. Soc. 35, 1666 (1913)
J.W. Beams, C.W. Hulburt, W.E. Lotz, R.M. Montague, Rev. Sci. Instrum. 26, 1181 (1955)
J.W. Beams, A.M. Clarke, Rev. Sci. Instrum. 33, 750 (1962)
W.M. Haynes, M.J. Hiza, N.V. Frederick, Rev. Sci. Instrum. 47, 1237 (1976)
R. Masui, W.M. Haynes, R.F. Chang, H.A. Davis, J.M.H.L. Sengers, Rev. Sci. Instrum. 55, 1132 (1984)
R. Masui, Int. J. Thermophys. 23, 921 (2002)
H. Wolf, H. Bettin, A. Gluschko, Meas. Sci. Technol. 17, 2581 (2006)
M. O. McLinden, NIST 73 (2014).
J.W. Clark, Rev. Sci. Instrum. 18, 915 (1947)
Th. Gast, “Microweighing in vacuo with a magnetic suspension balance” in Vacuum Microbalance Techniques. Volume 3. Proceedings of the Los Angeles Conference, K H Behrndt (Plenum Press, New York, 1963), pp. 45–54.
Th. Gast, ACTA IMEKO 4, 159 (1967)
Th. Gast, Naturwissenschaften 56, 434 (1969)
H. Sabrowsky, H.G. Deckert, Chem.-Ing.-Tech. 50, 217 (1978)
H. Sabrowsky, H.G. Deckert, Chem.-Ing.-Tech. 51, 880 (1979)
H.-W. Lösch, Entwicklung und Aufbau von neuen Magnetschwebewaagen zur berührungsfreien Messung vertikaler Kräfte (VDI Verlag, Fortschritt-Berichte VDI, Reihe 3, Nr. 138, Düsseldorf, 1987).
R. Kleinrahm, Entwicklung und Aufbau einer Dichtemessanlage zur Messung der Siede- und Taudichten reiner fluider Stoffe auf der gesamten Phasengrenzkurve, Dissertation, Ruhr-Universität Bochum, 1983.
R. Kleinrahm and W. Wagner, Entwicklung und Aufbau einer Dichtemeßanlage zur Messung der Siede- und Taudichten reiner fluider Stoffe auf der gesamten Phasengrenzkurve (VDI Verlag, Fortschritt-Berichte VDI, Reihe 3, Nr. 92, Düsseldorf, 1984).
R. Kleinrahm, W. Wagner, J. Chem. Thermodyn. 18, 739 (1986)
H.W. Lösch, R. Kleinrahm, W. Wagner, Chem. Ing. Tec. 66, 1055 (1994)
H. W. Loesch, R. Kleinrahm, W. Wagner, “Neue Magnetschwebewaagen für gravimetrische Messungen in der Verfahrenstechnik” in Jahrbuch 1994 Verfahrenstechnik Und Chemieingenieur Wesen (VDI Vlg. Düsseldorf, 1994), pp. 117–137.
F. Dreisbach, H.W. Lösch, J. Therm. Anal. Calorim. 62, 515 (2000)
R. Kleinrahm, X. Yang, M.O. McLinden, M. Richter, Adsorption 25, 717 (2019)
X. Yang, M. Richter, Ind. Eng. Chem. Res. 59, 3238 (2020)
W. Wagner, R. Kleinrahm, Metrologia 41, S24 (2004)
X. Yang, M. Richter, Z. Wang, Z. Li, J. Chem. Thermodyn. 91, 17 (2015)
R. Kleinrahm, W. Duschek, W. Wagner, J. Chem. Thermodyn. 18, 1103 (1986)
R. Kleinrahm, W. Duschek, W. Wagner, M. Jaeschke, J. Chem. Thermodyn. 20, 621 (1988)
G. Händel, R. Kleinrahm, W. Wagner, J. Chem. Thermodyn. 24, 685 (1992)
W. Duschek, R. Kleinrahm, W. Wagner, J. Chem. Thermodyn. 22, 827 (1990)
W. Duschek, R. Kleinrahm, W. Wagner, J. Chem. Thermodyn. 22, 841 (1990)
R. Gilgen, R. Kleinrahm, W. Wagner, J. Chem. Thermodyn. 24, 1243 (1992)
G. Händel, R. Kleinrahm, W. Wagner, J. Chem. Thermodyn. 24, 697 (1992)
W. Duschek, R. Kleinrahm, W. Wagner, M. Jaeschke, J. Chem. Thermodyn. 20, 1069 (1988)
P. Nowak, R. Kleinrahm, W. Wagner, J. Chem. Thermodyn. 29, 1137 (1997)
P. Nowak, R. Kleinrahm, W. Wagner, J. Chem. Thermodyn. 29, 1157 (1997)
R. Gilgen, R. Kleinrahm, W. Wagner, J. Chem. Thermodyn. 26, 399 (1994)
R. Gilgen, R. Kleinrahm, W. Wagner, J. Chem. Thermodyn. 26, 383 (1994)
P. Nowak, R. Kleinrahm, W. Wagner, J. Chem. Thermodyn. 28, 1423 (1996)
P. Nowak, R. Kleinrahm, W. Wagner, J. Chem. Thermodyn. 28, 1441 (1996)
M. Funke, R. Kleinrahm, W. Wagner, J. Chem. Thermodyn. 34, 735 (2002)
R. Gilgen, R. Kleinrahm, W. Wagner, J. Chem. Thermodyn. 24, 953 (1992)
M. Funke, R. Kleinrahm, W. Wagner, J. Chem. Thermodyn. 34, 717 (2002)
M. Funke, R. Kleinrahm, W. Wagner, J. Chem. Thermodyn. 34, 2001 (2002)
M. Funke, R. Kleinrahm, W. Wagner, J. Chem. Thermodyn. 34, 2017 (2002)
S. Glos, R. Kleinrahm, W. Wagner, J. Chem. Thermodyn. 36, 1037 (2004)
N. Pieperbeck, R. Kleinrahm, W. Wagner, Entwicklung und Aufbau einer Präzisions-Gasdichtemeßanlage zur Bestimmung der Dichte von Erdgasen (VDI-Verlag, Fortschr.-Ber. VDI Reihe 3 Nr. 228., Dusseldorf, 1990).
N. Pieperbeck, R. Kleinrahm, W. Wagner, M. Jaeschke, J. Chem. Thermodyn. 23, 175 (1991)
M. Richter, R. Kleinrahm, S. Glos, W. Wagner, R. Span, P. Schley, M. Uhrig, Int. J. Thermophys. 31, 680 (2010)
M. Richter, M.A.B. Souissi, R. Span, P. Schley, J. Chem. Eng. Data 59, 2021 (2014)
X. Yang, M. Richter, M.A.B. Souissi, R. Kleinrahm, R. Span, J. Chem. Eng. Data 61, 2676 (2016)
M.A.B. Souissi, M. Richter, X. Yang, R. Kleinrahm, R. Span, J. Chem. Eng. Data 62, 362 (2017)
C. W. Scholz, Thermodynamic Properties of Liquids and Gas Mixtures as Contribution to a Sustainable Energy Supply, Dissertation, Ruhr-Universität Bochum (2021), https://hss-opus.ub.ruhr-uni-bochum.de/opus4/frontdoor/index/index/year/2021/docId/8270
M.A.B. Souissi, R. Kleinrahm, X. Yang, M. Richter, J. Chem. Eng. Data 62, 2973 (2017)
X. Yang, M.A.B. Souissi, R. Kleinrahm, M. Richter, J. Chem. Thermodyn. 122, 204 (2018)
M.O. McLinden, C. Lösch-Will, J. Chem. Thermodyn. 39, 507 (2007)
M.O. McLinden, Meas. Sci. Technol. 17, 2597 (2006)
M.O. McLinden, J. Chem. Eng. Data 54, 3181 (2009)
M. O. McLinden and E. W. Lemmon, NIST 1 (2010).
M.O. McLinden, M. Thol, E.W. Lemmon, NIST 2189, 1 (2010)
M. Richter, M.O. McLinden, E.W. Lemmon, J. Chem. Eng. Data 56, 3254 (2011)
M.O. McLinden, J. Chem. Eng. Data 56, 606 (2011)
M.E. Mondéjar, M.O. McLinden, E.W. Lemmon, J. Chem. Eng. Data 60, 2477 (2015)
M.O. McLinden, R.A. Perkins, E.W. Lemmon, T.J. Fortin, J. Chem. Eng. Data 60, 3646 (2015)
M.O. McLinden, M. Richter, J. Chem. Thermodyn. 99, 105 (2016)
M.O. McLinden, R. Akasaka, J. Chem. Eng. Data 65, 4201 (2020)
A.J. Rowane, E.G. Rasmussen, M.O. McLinden, J. Chem. Eng. Data 67, 3022 (2022)
Y. Kayukawa, Y. Kano, K. Fujii, H. Sato, Metrologia 49, 513 (2012)
K. Brachthäuser, R. Kleinrahm, H.-W. Loesch, W. Wagner, Entwicklung eines neuen DichtemeBverfahrens und Aufbau einer Hochtemperatur-Hochdruck-DichtemeBanlage (VDI-Verlag, Fortschr.-Ber. VDI Reihe 8 Nr. 371, Dusseldorf, 1993).
W. Wagner, K. Brachthäuser, R. Kleinrahm, H.W. Lösch, Int. J. Thermophys. 16, 399 (1995)
J. Klimeck, R. Kleinrahm, W. Wagner, J. Chem. Thermodyn. 30, 1571 (1998)
J. Klimeck, R. Kleinrahm, W. Wagner, J. Chem. Thermodyn. 33, 251 (2001)
P. Claus, R. Kleinrahm, W. Wagner, J. Chem. Thermodyn. 35, 159 (2003)
G. Schilling, R. Kleinrahm, W. Wagner, J. Chem. Thermodyn. 40, 1095 (2008)
D. Sommer, R. Kleinrahm, R. Span, W. Wagner, J. Chem. Thermodyn. 43, 117 (2011)
A. Docter, H.-W. Lösch, W. Wagner, Entwicklung und Aufbau einer Anlage zur simultanen Messung der Viskosität und der Dichte fluider Stoffe (VDI-Verlag, Fortschr.-Ber. VDI, Reihe 3, Nr. 494, Dusseldorf, 1997).
A. Docter, H.W. Lösch, W. Wagner, Int. J. Thermophys. 20, 485 (1999)
C. Evers, H.W. Loesch, W. Wagner, Int. J. Thermophys. 23, 1411 (2002)
M. Schäfer, M. Richter, R. Span, J. Chem. Thermodyn. 89, 7 (2015)
M. Schäfer, Improvements to Two Viscometers Based on a Magnetic Suspension Coupling and Measurements on Carbon Dioxide, Dissertation, Ruhr-Universität Bochum (2016), https://hss-opus.ub.ruhr-uni-bochum.de/opus4/frontdoor/index/index/docId/4542.
R. Wegge, Thermodynamic Properties of the (Argon + Carbon Dioxide) System, Dissertation, Ruhr-Universität Bochum (2016), https://hss-opus.ub.ruhr-uni-bochum.de/opus4/frontdoor/index/index/docId/4716.
K. Humberg, Viscosity Measurements of Binary Gas Mixtures and Analysis of Approaches for the Modeling of Mixture Viscosities, Dissertation, Ruhr-Universität Bochum (2020), https://hss-opus.ub.ruhr-uni-bochum.de/opus4/frontdoor/index/index/year/2020/docId/7710.
H. Gedanitz, Schallgeschwindigkeits- und Dichtemessungen in Fluiden, Dissertation, Ruhr-Universität Bochum (2010), https://hss-opus.ub.ruhr-uni-bochum.de/opus4/frontdoor/index/index/year/2018/docId/2374
N. Kuramoto, K. Fujii, A. Waseda, Metrologia 41, S84 (2004)
T. Kimura, Y. Kayukawa, K. Saito, J. Chem. Eng. Data 62, 1422 (2017)
Y. Kayukawa, Int. J. Refrig. 119, 349 (2020)
P. V. Patil, Commissioning of a Magnetic Suspension Densitometer for High-Accuracy Density Measurements of Natural Gas Mixtures, Dissertation, Texas A&M University (2005), http://oaktrust.library.tamu.edu/handle/1969.1/ETD-TAMU-1003.
P. Patil, S. Ejaz, M. Atilhan, D. Cristancho, J.C. Holste, K.R. Hall, J. Chem. Thermodyn. 39, 1157 (2007)
M. Atilhan, High Accuracy P-ρ-t Measurements up to 200 MPa between 200 K and 500 K Using a Compact Single Sinker Magnetic Suspension Densimeter for Pure and Natural Gas like Mixtures, Dissertation, Texas A&M University (2007).
M. Atilhan, S. Ejaz, J. Zhou, D. Cristancho, I. Mantilla, J. Holste, K.R. Hall, J. Chem. Eng. Data 55, 4907 (2010)
M. Atilhan, S. Aparicio, S. Ejaz, D. Cristancho, K.R. Hall, J. Chem. Eng. Data 56, 212 (2011)
M. Atilhan, S. Aparicio, S. Ejaz, D. Cristancho, I. Mantilla, K.R. Hall, J. Chem. Eng. Data 56, 3766 (2011)
M. Atilhan, S. Aparicio, S. Ejaz, J. Zhou, M. Al-Marri, J.J. Holste, K.R. Hall, J. Chem. Thermodyn. 82, 134 (2015)
I.D. Mantilla, D.E. Cristancho, S. Ejaz, K.R. Hall, M. Atilhan, G.A. Iglesias-Silva, J. Chem. Eng. Data 55, 4611 (2010)
D.E. Cristancho, I.D. Mantilla, S. Ejaz, K.R. Hall, M. Atilhan, G.A. Iglesias-Silva, J. Chem. Eng. Data 55, 2746 (2010)
I.D. Mantilla, D.E. Cristancho, S. Ejaz, K.R. Hall, M. Atilhan, G.A. Iglesias-Silva, J. Chem. Eng. Data 55, 4227 (2010)
D.E. Cristancho, I.D. Mantilla, S. Ejaz, K.R. Hall, M. Atilhan, G.A. Iglesia-Silva, J. Chem. Eng. Data 55, 826 (2010)
M.E. Mondéjar, J.J. Segovia, C.R. Chamorro, Measurement 44, 1768 (2011)
C.R. Chamorro, J.J. Segovia, M.C. Martín, M.A. Villamañán, J.F. Estela-Uribe, J.P.M. Trusler, J. Chem. Thermodyn. 38, 916 (2006)
M.E. Mondéjar, M.A. Villamañán, R. Span, C.R. Chamorro, J. Chem. Eng. Data 56, 3933 (2011)
M.E. Mondéjar, M.C. Martín, R. Span, C.R. Chamorro, J. Chem. Thermodyn. 43, 1950 (2011)
M.E. Mondéjar, R.M. Villamañán, R. Span, C.R. Chamorro, J. Chem. Thermodyn. 48, 254 (2012)
M.E. Mondéjar, T.E. Fernández-Vicente, F. Haloua, C.R. Chamorro, J. Chem. Eng. Data 57, 2581 (2012)
R. Hernández-Gómez, D. Tuma, J.J. Segovia, C.R. Chamorro, J. Chem. Thermodyn. 96, 1 (2016)
R. Hernández-Gómez, D. Tuma, R. Villamañán, C.R. Chamorro, J. Chem. Thermodyn. 101, 168 (2016)
H. Li, M. Gong, H. Zhang, X. Dong, J. Wu, J. Chem. Thermodyn. 78, 225 (2014)
H. Zhang, M. Gong, H. Li, H. Guo, X. Dong, J. Wu, J. Chem. Thermodyn. 81, 60 (2015)
H. Li, X. Dong, H. Zhang, Q. Zhong, H. Guo, Y. Zhao, J. Shen, M. Gong, Int. J. Refrig. 85, 376 (2018)
H. Zhang, Q. Zhong, M. Gong, H. Li, X. Dong, J. Shen, J. Wu, J. Chem. Eng. Data 61, 3241 (2016)
H. Zhang, M. Gong, H. Li, H. Guo, X. Dong, J. Wu, Fluid Phase Equilib. 408, 232 (2016)
M. Gong, H. Zhang, H. Li, Q. Zhong, X. Dong, J. Shen, J. Wu, Int. J. Refrig. 64, 168 (2016)
Q. Zhong, X. Dong, H. Zhang, H. Li, M. Gong, J. Shen, J. Wu, J. Chem. Thermodyn. 107, 126 (2017)
Q. Zhong, H. Li, X. Dong, H. Zhang, Y. Zhao, H. Guo, J. Shen, M. Gong, J. Chem. Thermodyn. 118, 77 (2018)
H. Zhang, X. Dong, Q. Zhong, H. Li, M. Gong, J. Shen, J. Wu, Int. J. Refrig. 73, 144 (2017)
X.-D. Cai, N. Zhang, L.-X. Chen, P. Hu, G. Zhao, M.-H. Liu, Fluid Phase Equilib. 460, 119 (2018)
H. Zhang, H. Li, B. Gao, Q. Zhong, W. Wu, W. Liu, X. Dong, M. Gong, E. Luo, Int. J. Refrig. 95, 28 (2018)
X. Yang, Z. Wang, Z. Li, J. Chem. Eng. Data 60, 3353 (2015)
X. Yang, Z. Wang, Z. Li, Fluid Phase Equilib. 418, 94 (2016)
M. Richter, R. Kleinrahm, R. Lentner, R. Span, J. Chem. Thermodyn. 93, 205 (2016)
R. Lentner, M. Richter, R. Kleinrahm, R. Span, J. Chem. Thermodyn. 112, 68 (2017)
R. Lentner, P. Eckmann, R. Kleinrahm, R. Span, M. Richter, J. Chem. Thermodyn. 142, 106002 (2020)
P. Eckmann, N. von Preetzmann, G. Cavuoto, J. Li, A. van der Veen, R. Kleinrahm, M. Richter, Int. J. Thermophys. 41, 156 (2020)
G. Cavuoto, N. von Preetzmann, P. Eckmann, J. Li, A.M.H. van der Veen, R. Kleinrahm, M. Richter, Int. J. Thermophys. 42, 43 (2021)
N. von Preetzmann, R. Kleinrahm, P. Eckmann, G. Cavuoto, M. Richter, Int. J. Thermophys. 42, 127 (2021)
A. Karimi, T.J. Hughes, M. Richter, E.F. May, J. Chem. Eng. Data 61, 2782 (2016)
X. Yang, R. Kleinrahm, M.O. McLinden, M. Richter, Adsorption 26, 645 (2020)
C. Tietz, M. Sekulla, X. Yang, R. Schmid, M. Richter, Ind. Eng. Chem. Res. 59, 13283 (2020)
K. Moritz, R. Kleinrahm, M.O. McLinden, M. Richter, Meas. Sci. Technol. 28, 127004 (2017)
K. Moritz, Dew-Point Densities of Fluid Mixtures—New Approaches for Measurement as the Key to Precision, Dissertation, Ruhr-Universität Bochum (2020), https://hss-opus.ub.ruhr-uni-bochum.de/opus4/frontdoor/index/index/year/2020/docId/7626.
H. Watanabe, N. Yamada, M. Okaji, Int. J. Thermophys. 25, 221 (2004)
M.O. McLinden, J.D. Splett, J. Res. Natl. Inst. Stand. Technol. 113, 29 (2008)
M. Richter, M.O. McLinden, Sci. Rep. 7, 6185 (2017)
L. Börnstein, Elastic Constants of Second Order: Temperature Coefficients Tc (Springer, New York, 2001).
J.J. Hall, Phys. Rev. 161, 756 (1967)
H. Lösch, R. Kleinrahm, W. Wagner, Jahrbuch 1994: Verfahrenstechnik und Chemieingenieurwesen (VDI-Verlag, Dusseldorf, 1994)
N. Vlajic, M. Davis, C. Stambaugh, J. Dyn. Syst. Meas. Control 140, (2018).
C.A. Stambaugh, NIST 6, 75 (2017)
E.C. Benck, C.A. Stambaugh, E.C. Mulhern, P.J. Abbott, Z.J. Kubarych, Acta IMEKO 6, 70 (2017)
E.F. May, R.C. Miller, Z. Shan, J. Chem. Eng. Data 46, 1160 (2001)
L. Bernardini, A. Maistrovoi, M. Sekulla, M. Mehring, M. Armbrüster, M. Richter, J. Chem. Eng. Data 67, 1663 (2022)
E. S. Pouya, Advancing Methane-Nitrogen Separation with Zeolitic Adsorbents, Dissertation, The University Western Australia, 2023.
S. Rahman, A. Arami-Niya, X. Yang, G. Xiao, G.K. Li, E.F. May, Commun. Chem. 3, 1 (2020)
X. Yang, A. Arami-Niya, G. Xiao, E.F. May, Langmuir 36, 14967 (2020)
M. Richter, R. Kleinrahm, J. Chem. Thermodyn. 74, 58 (2014)
E. W. Lemmon, I. H. Bell, M. L. Huber, M. O. McLinden, NIST Standard Reference Database 23: Reference Fluid Thermodynamic and Transport Properties-REFPROP, Version 10.0, National Institute of Standards and Technology (2018).
R. Span, R. Beckmüller, S. Hielscher, A. Jäger, E. Mickoleit, T. Neumann, S. Pohl, TREND. Thermodynamic Reference and Engineering Data 5.0 (Bochum, Germany: Lehrstuhl für Thermodynamik, Ruhr-Universität Bochum, 2020).
I.H. Bell, J. Wronski, S. Quoilin, V. Lemort, Ind. Eng. Chem. Res. 53, 2498 (2014)
A. Polt, B. Platzer, G. Maurer, Chem. Tech. (Leipzig) 44, 216 (1992)
E.W. Lemmon, R. Span, J. Chem. Eng. Data 51, 785 (2006)
R. Tillner-Roth, A. Yokozeki, J. Phys. Chem. Ref. Data 26, 1273 (1997)
R. Span, W. Wagner, Int. J. Thermophys. 24, 41 (2003)
U. Setzmann, W. Wagner, J. Phys. Chem. Ref. Data 20, 1061 (1991)
R. Span, W. Wagner, J. Phys. Chem. Ref. Data 25, 1509 (1996)
D.O. Vega, K. Hall, J. Holste, A.H. Harvey, E.W. Lemmon, An Equation of State for the Thermodynamic Properties of Helium, NIST Interagency/Internal Report (NISTIR) 8474 (National Institute of Standards and Technology, Gaithersburg, MD, 2023)
M.R. Moldover, M.O. McLinden, J. Chem. Thermodyn. 42, 1193 (2010)
R. Span, E.W. Lemmon, R.T. Jacobsen, W. Wagner, A. Yokozeki, J. Phys. Chem. Ref. Data 29, 1361 (2000)
Ch. Tegeler, R. Span, W. Wagner, J. Phys. Chem. Ref. Data 28, 779 (1999)
J. Smukala, R. Span, W. Wagner, J. Phys. Chem. Ref. Data 29, 1053 (2000)
C. Guder, W. Wagner, J. Phys. Chem. Ref. Data 38, 33 (2009)
D. Bücker, W. Wagner, J. Phys. Chem. Ref. Data 35, 205 (2006)
E.W. Lemmon, M.O. McLinden, W. Wagner, J. Chem. Eng. Data 54, 3141 (2009)
D. Bücker, W. Wagner, J. Phys. Chem. Ref. Data 35, 929 (2006)
V. Marx, A. Pruß, W. Wagner, Neue Zustandsgleichungen für R12, R22, R11 und R113: Beschreibung des thermodynamischen Zustandsverhaltens bei Temperaturen bis 525 K und Drücken bis 200 MPa (VDI-Verlag, Düsseldorf, 1992)
A. Kamei, S.W. Beyerlein, R.T. Jacobsen, Int. J. Thermophys. 16, 1155 (1995)
M. Thol, E.W. Lemmon, Int. J. Thermophys. 37, 28 (2016)
Y. Zhou, J. Liu, S.G. Penoncello, E.W. Lemmon, J. Phys. Chem. Ref. Data 43, 043105 (2014)
J.A. Schroeder, S.G. Penoncello, J.S. Schroeder, J. Phys. Chem. Ref. Data 43, 043102 (2014)
O. Kunz, W. Wagner, J. Chem. Eng. Data 57, 3032 (2012)
J. Gernert, R. Span, J. Chem. Thermodyn. 93, 274 (2016)
M. Thol, M. Richter, E.F. May, E.W. Lemmon, R. Span, J. Phys. Chem. Ref. Data 48, 033102 (2019)
N.P. Patel, J.M. Zielinski, J. Samseth, R.J. Spontak, Macromol. Chem. Phys. 205, 2409 (2004)
S. Janakiram, L. Ansaloni, S.-A. Jin, X. Yu, Z. Dai, R.J. Spontak, L. Deng, Green Chem. 22, 3546 (2020)
A.V. Talyzin, A. Jacob, J. Alloys Compd. 395, 154 (2005)
G. Mercier, A. Klechikov, M. Hedenström, D. Johnels, I.A. Baburin, G. Seifert, R. Mysyk, A.V. Talyzin, J. Phys. Chem. C 119, 27179 (2015)
S. Cotugno, E. Di Maio, G. Mensitieri, S. Iannace, G.W. Roberts, R.G. Carbonell, H.B. Hopfenberg, Ind. Eng. Chem. Res. 44, 1795 (2005)
L. Sorrentino, E. Di Maio, S. Iannace, J. Appl. Polym. Sci. 116, 27 (2010)
Y. Zhong, R.E. Critoph, R.N. Thorpe, Z. Tamainot-Telto, Yu.I. Aristov, Appl. Therm. Eng. 27, 2455 (2007)
Y. Zhong, R.E. Critoph, R.N. Thorpe, Z. Tamainot-Telto, Appl. Therm. Eng. 29, 1180 (2009)
S. Ottiger, R. Pini, G. Storti, M. Mazzotti, Langmuir 24, 9531 (2008)
A. Streb, M. Mazzotti, Adsorption 27, 541 (2021)
A.M. Ribeiro, T.P. Sauer, C.A. Grande, R.F.P.M. Moreira, J.M. Loureiro, A.E. Rodrigues, Ind. Eng. Chem. Res. 47, 7019 (2008)
I.C. Ferreira, T.J. Ferreira, A.D.S. Barbosa, B. de Castro, R.P.P.L. Ribeiro, J.P.B. Mota, V.D. Alves, L. Cunha-Silva, I.A.A.C. Esteves, L.A. Neves, Sep. Purif. Technol. 276, 119303 (2021)
I.A.A.C. Esteves, M.S.S. Lopes, P.M.C. Nunes, J.P.B. Mota, Sep. Purif. Technol. 62, 281 (2008)
J.E. Sosa, C. Malheiro, P.J. Castro, R.P.P.L. Ribeiro, M.M. Piñeiro, F. Plantier, J.P.B. Mota, J.M.M. Araújo, A.B. Pereiro, Global Chall. 7, 2200107 (2023)
P. Chowdhury, C. Bikkina, S. Gumma, J. Phys. Chem. C 113, 6616 (2009)
P. Mishra, S. Edubilli, B. Mandal, S. Gumma, J. Phys. Chem. C 118, 6847 (2014)
Y. Belmabkhout, R. Serna-Guerrero, A. Sayari, Ind. Eng. Chem. Res. 49, 359 (2010)
A. Sayari, Y. Belmabkhout, E. Da’na, Langmuir 28, 4241 (2012)
B. Yu, H. Cong, X.S. Zhao, Z. Chen, Adsorpt. Sci. Technol. 29, 781 (2011)
H. Cong, B. Yu, J. Tang, X.S. Zhao, J. Polym. Res. 19, 9761 (2012)
I. Bottero, B. Bonelli, S.E. Ashbrook, P.A. Wright, W. Zhou, M. Tagliabue, M. Armandi, E. Garrone, Phys. Chem. Chem. Phys. 13, 744 (2011)
M. Tagliabue, C. Rizzo, N.B. Onorati, E.F. Gambarotta, A. Carati, F. Bazzano, Fuel 93, 238 (2012)
F. Karadas, C.T. Yavuz, S. Zulfiqar, S. Aparicio, G.D. Stucky, M. Atilhan, Langmuir 27, 10642 (2011)
T. Altamash, M. Atilhan, A. Aliyan, R. Ullah, M. Nasser, S. Aparicio, Chem. Eng. Technol. 40, 778 (2017)
Y. Tang, X. Wang, Y. Wen, X. Zhou, Z. Li, Ind. Eng. Chem. Res. 59, 6219 (2020)
S. Du, J. Huang, A.W. Anjum, J. Xiao, Z. Li, J. Mater. Chem. A 9, 23873 (2021)
L. Du, G. Mauer, R. Vaßen, Energy Procedia 29, 147 (2012)
L. Du, G. Mauer, R. Vaßen, Metall. Mater. Trans. E 2, 50 (2015)
B.B. Saha, I.I. El-Sharkawy, R. Thorpe, R.E. Critoph, Int. J. Refrig. 35, 499 (2012)
K. Uddin, I.I. El-Sharkawy, T. Miyazaki, B.B. Saha, S. Koyama, H.-S. Kil, J. Miyawaki, S.-H. Yoon, Appl. Therm. Eng. 72, 211 (2014)
P. Sharma, J. Yeo, M.H. Han, C.H. Cho, J. Mater. Chem. A 1, 2602 (2013)
Y.J. Kim, M.-Z. Kim, S.F. Alam, A. Rehman, A. Devipriyanka, P. Sharma, H.R. Lee, C.-H. Cho, Mater. Chem. Phys. 259, 124021 (2021)
H. Yanagihara, K. Yamashita, A. Endo, H. Daiguji, J. Phys. Chem. C 117, 21795 (2013)
J. Hwang, K. Yanagita, K. Sakamoto, W.-L. Hsu, S. Kataoka, A. Endo, H. Daiguji, Langmuir 35, 10762 (2019)
M. Gonzalez-Miquel, J. Bedia, C. Abrusci, J. Palomar, F. Rodriguez, J. Phys. Chem. B 117, 3398 (2013)
J. Lemus, C. Paramio, D. Hospital-Benito, C. Moya, R. Santiago, J. Palomar, ACS Sustain. Chem. Eng. 10, 13917 (2022)
A.L. Ammitzbøll, S. Lysgaard, A. Klukowska, T. Vegge, U.J. Quaade, J. Chem. Phys. 138, 164701 (2013)
A. Bialy, P.B. Jensen, D. Blanchard, T. Vegge, U.J. Quaade, J. Solid State Chem. 221, 32 (2015)
R. Jiang, T. Fuller, S. Brawn, C. Gittleman, Electrochim. Acta 110, 306 (2013)
I.A. Richardson, J.W. Leachman, T.M. Blackham, S.G. Penoncello, AIP Conf. Proc. 1573, 1086 (2014)
C. Moya, J. Palomar, M. Gonzalez-Miquel, J. Bedia, F. Rodriguez, Ind. Eng. Chem. Res. 53, 13782 (2014)
R.-Y. Lin, L. Deng, D.-L. An, Z.-H. Zhou, Dalton Trans. 52, 562 (2023)
M.M. Khan, G. Bengtson, S. Neumann, M.M. Rahman, V. Abetz, V. Filiz, RSC Adv. 4, 32148 (2014)
A. Raje, P. Georgopanos, J. Koll, J. Lillepärg, U.A. Handge, V. Abetz, Polymers 15, 118 (2023)
A.A.K. Firas, A.M. Shariff, L.K. Keong, N. Mellon, Appl. Mech. Mater. 625, 600 (2014)
F.A.A. Kareem, A.M. Shariff, S. Ullah, N. Mellon, L.K. Keong, Microporous Mesoporous Mater. 267, 221 (2018)
Y. Huang, B. Zhang, J. Duan, W. Liu, X. Zheng, L. Wen, X. Ke, D. Li, Cryst. Growth Des. 14, 2866 (2014)
Z. Zhang, B. Yang, B. Zhang, M. Cui, J. Tang, X. Qiao, Nat. Commun. 13, 2353 (2022)
H. Zhou, X. Liu, J. Zhang, X. Yan, Y. Liu, A. Yuan, Int. J. Hydrog. Energy 39, 2160 (2014)
H. Jung, J. Yuh, S. Cho, W. Lee, J. Alloys Compd. 601, 63 (2014)
H. Jung, S. Cho, W. Lee, J. Alloys Compd. 635, 203 (2015)
P.K. Prabhakaran, J. Deschamps, J. Mater. Chem. A 3, 7014 (2015)
P.K. Prabhakaran, L. Catoire, J. Deschamps, Microporous Mesoporous Mater. 243, 214 (2017)
C.G. Piscopo, A. Polyzoidis, M. Schwarzer, S. Loebbecke, Microporous Mesoporous Mater. 208, 30 (2015)
M. Winkler, J. Schipper, C. Teicht, P. Corhan, A. Polyzoidis, K. Bartholomé, O. Schäfer-Welsen, S. Pappert, Energies 15, 3271 (2022)
X. Duan, H. Wang, Y. Cui, Y. Yang, Z. Wang, B. Chen, G. Qian, RSC Adv. 5, 84446 (2015)
X. Wan, K. Zhang, T. Wan, Y. Yan, Z. Ye, X. Peng, (2022).
Q. Wang, Y. Liu, J. Chen, Z. Du, J. Mi, Environ. Sci. Technol. 50, 7879 (2016)
J. Deng, W. Zou, J. Mi, Z. Du, P. Kong, C. Zhang, Ind. Eng. Chem. Res. 60, 7624 (2021)
J. Breternitz, Y.E. Vilk, E. Giraud, H. Reardon, T.K.A. Hoang, A. Godula-Jopek, D.H. Gregory, Chemsuschem 9, 1312 (2016)
Y. Pang, M. Y. Soliman, J. Sheng, Investigation of Adsorption Effects on Nanopores in Shale Gas Reservoir by Simplified Local-Density Model (OnePetro, 2016).
J. He, J.W.F. To, P.C. Psarras, H. Yan, T. Atkinson, R.T. Holmes, D. Nordlund, Z. Bao, J. Wilcox, Adv. Energy Mater. 6, 1502491 (2016)
S. Chen, H. Gong, B. Dindoruk, J. He, Z. Bao, A.C.S. Appl, Nano Mater. 3, 8278 (2020)
C. Seibel, C. Wedler, N. Vorobiev, M. Schiemann, V. Scherer, R. Span, T.M. Fieback, Fuel Process. Technol. 153, 81 (2016)
C. Wedler, R. Span, Chem. Eng. Sci. 231, 116281 (2021)
M. Kudasik, Meas. Sci. Technol. 27, 035903 (2016)
P.P. Ziemiański, A. Derkowski, Int. J. Hydrog. Energy 47, 28794 (2022)
S. Simonato, J. Möllmer, M. Lange, R. Gläser, R. Staudt, C. Feldmann, RSC Adv. 6, 12446 (2016)
I. Dönges, M.I. Büschges, C. Njel, J.J. Schneider, Dalton Trans. 51, 13725 (2022)
Z. Lu, J. Bai, C. Hang, F. Meng, W. Liu, Y. Pan, X. You, Chem. Eur. J. 22, 6277 (2016)
L. Pan, X. Xiao, H. Tian, Q. Zhou, P. Cheng, Mar. Pet. Geol. 73, 433 (2016)
H. Gai, T. Li, X. Wang, H. Tian, X. Xiao, Q. Zhou, Mar. Pet. Geol. 120, 104565 (2020)
J. Zhong, G. Chen, C. Lv, W. Yang, Y. Xu, S. Yang, L. Xue, Nat. Gas Geosci. 1, 165 (2016)
J. Li, P. Li, S. Zhou, Z. Sun, B. Meng, Y. Li, Chem. Eng. J. 444, 136617 (2022)
S.-H. Hwang, W.M. Choi, S.K. Lim, Mater. Lett. 167, 18 (2016)
J. Liu, Y. Yao, Z. Zhu, L. Cheng, G. Wang, Energy Explor. Exploit. 34, 527 (2016)
T. Wang, D. Hu, A. Jia, T. Dong, Y. Hou, S. He, M. Chen, X. Guo, Q. He, Y. Zeng, R. Yang, Energy Fuels 36, 1531 (2022)
T. Ncube, K.S. Reddy, A. Al Shoaibi, C. Srinivasakannan, Energy Fuels 31, 1882 (2017)
P. Azhagapillai, M. Khaleel, F. Zoghieb, G. Luckachan, L. Jacob, D. Reinalda, ACS Omega 7, 6463 (2022)
R. Lou, Q. Dong, H. Nie, Energy Fuels 31, 1100 (2017)
Z. Jiang, D. Zhang, J. Zhao, Y. Zhou, J. Nat. Gas Eng. 46, 436 (2017)
T. Wu, J. Zhao, W. Zhang, D. Zhang, Nano Energy 69, 104426 (2020)
G. Gadikota, B. Dazas, G. Rother, M.C. Cheshire, I.C. Bourg, J. Phys. Chem. C 121, 26539 (2017)
H. Zhao, Z. Lai, A. Firoozabadi, Sci. Rep. 7, 1 (2017)
T. Wu, H. Zhao, S. Tesson, A. Firoozabadi, Fuel 235, 855 (2019)
V.E. Strempel, R.N. d’Alnoncourt, M. Driess, F. Rosowski, Rev. Sci. Instrum. 88, 074102 (2017)
A. Arami-Niya, T.E. Rufford, G. Birkett, Z. Zhu, Microporous Mesoporous Mater. 244, 218 (2017)
G. Feng, Y. Zhu, S. Chen, Y. Wang, W. Ju, Y. Hu, Z. You, G.G.X. Wang, Energy Fuels 34, 3121 (2020)
Z. Lu, J. Zhang, H. He, L. Du, C. Hang, Inorg. Chem. Front. 4, 736 (2017)
L. Du, Z. Lu, L. Xu, J. Zhang, RSC Adv. 7, 21268 (2017)
X. Tang, Z. Jiang, S. Jiang, L. Cheng, Y. Zhang, Fuel 187, 285 (2017)
C. Qin, Y. Jiang, Y. Fu, S. Chen, X. Song, S. Zuo, D. Wu, N. Zou, Energy 263, 125898 (2023)
G. Makhloufi, B. Francis, J. Dechnik, A. Strzelczyk, C. Janiak, Polyhedron 127, 59 (2017)
C. Jansen, N. Tannert, D. Lenzen, M. Bengsch, S. Millan, A. Goldman, D.N. Jordan, L. Sondermann, N. Stock, C. Janiak, Z. Anorg. Allg. Chem. 648, e202200170 (2022)
H. Cai, P. Li, Z. Ge, Y. Xian, D. Lu, Adsorpt. Sci. Technol. 36, 1687 (2018)
M. Chen, Y. Kang, T. Zhang, X. Li, K. Wu, Z. Chen, Fuel 228, 39 (2018)
J. Liu, Y. Wen, H. Yuan, L. Jiang, Z. Liu, Y. Chen, Z. Shui, Energy Sci. Eng. 222, 211440 (2023)
B. Zheng, L. Huang, X. Cao, S. Shen, H. Cao, C. Hang, W. Zeng, Z. Wang, CrystEngComm 20, 1874 (2018)
T. Cao, H. Liu, A. Pan, M. Deng, Q. Cao, Y. Yu, Y. Huang, Z. Xiao, Geofluids 2022, e2036451 (2022)
Z. Fan, J. Hou, X. Ge, P. Zhao, J. Liu, Energies 11, 3078 (2018)
Y. Wang, H.-F. Cheng, Q.-H. Hu, L.-B. Jia, X.-M. Wang, S.-S. Gao, L.-F. Liu, Pet. Sci. 20, 34 (2023)
L.H. de Oliveira, J.G. Meneguin, M.V. Pereira, E.A. da Silva, W.M. Grava, J.F. do Nascimento, P.A. Arroyo, Microporous Mesoporous Mater. 284, 247 (2019)
L.H. de Oliveira, M.V. Pereira, J.G. Meneguin, M.A.S.D. de Barros, J.F. Nascimento, P.A. Arroyo, J. CO2 Util. 67, 102296 (2023)
J. Hwang, R. Pini, Environ. Sci. Technol. 53, 11588 (2019)
J. Hwang, H. Azzan, R. Pini, C. Petit, J. Chem. Eng. Data 67, 1674 (2022)
A. Shamu, M. Dunnewold, H. Miedema, Z. Borneman, K. Nijmeijer, J. Supercrit. Fluids 144, 63 (2019)
E.B. Boz, M. Fritz, A. Forner-Cuenca, Adv. Mater. Interfaces 10, 2202497 (2023)
Y. Pang, Y. Tian, M.Y. Soliman, Y. Shen, Fuel 240, 192 (2019)
Y. Pang, X. Hu, S. Wang, S. Chen, M.Y. Soliman, H. Deng, Chem. Eng. J. 396, 125212 (2020)
J. Vargas Schmitz, M. Kriesten, M. Hartmann, M. Kaspereit, Sep. Purif. Technol. 217, 48 (2019).
K. Schmidt, L. Haas, A.L. Bidarouni, A. Reinhardt, F. Döpper, J. Franke, Procedia CIRP 107, 1373 (2022)
R. Thür, V. Lemmens, D. Van Havere, M. van Essen, K. Nijmeijer, I.F.J. Vankelecom, J. Membr. Sci. 610, 118195 (2020)
R. Thür, D.V. Havere, N.V. Velthoven, S. Smolders, A. Lamaire, J. Wieme, V.V. Speybroeck, D.D. Vos, I.F.J. Vankelecom, J. Mater. Chem. A 9, 12782 (2021)
M. Li, X. Yang, P. Connolly, N. Robinson, E.F. May, M. Mahmoud, A. El-Husseiny, M.L. Johns, Ind. Eng. Chem. Res. 59, 18280 (2020)
X. Han, X. Yang, C. Yu, S. Lu, E.S. Pouya, P. Bai, J. Lyu, X. Guo, CrystEngComm 23, 3026 (2021)
K. Yang, E. Sadeghi Pouya, L. Liu, M. Li, X. Yang, N. Robinson, E. F. May, M. L. Johns, ChemPhysChem e202100794 (2022).
N.A. Mohamad, N.A. Zubair, E.A. Lotf, M.M. Nasef, A. Ahmad, R.R. Ali, T.A.T. Abdullah, T.M. Ting, I.O.P. Conf, Ser. Mater. Sci. Eng. 808, 012029 (2020)
N.A. Zubair, M.M. Nasef, E.C. Abdullah, A. Ahmad, J. Phys. Conf. Ser. 2259, 012024 (2022)
K. Hu, H. Mischo, Energy Fuels 34, 2945 (2020)
K. Hu, V. Herdegen, H. Mischo, Fuel 318, 123666 (2022)
Y. Han, Y. Zhu, Y. Liu, Y. Wang, H. Zhang, W. Yu, Energies 13, 1690 (2020)
S.-K. Lee, H. Park, J.W. Yoon, K. Kim, S.J. Cho, G. Maurin, R. Ryoo, J.-S. Chang, A.C.S. Appl, Mater. Interfaces 12, 28484 (2020)
W. Qiang, L. Zhao, X. Gao, T. Liu, Z. Liu, W.-K. Yuan, D. Hu, J. Supercrit. Fluids 163, 104888 (2020)
X. Gao, Y. Chen, Z. Xu, L. Zhao, D. Hu, Ind. Eng. Chem. Res. 61, 13474 (2022)
O. Zheng, S. Luo, Q. Sun, S. Liu, S. Wei, Q. Xia, H. Ji, J. Hao, C. Deng, Innov. Food Sci. Emerg. Technol. 72, 102744 (2021)
M. Zhang, Z. Gong, Z. Tan, H. Liu, M. Yang, Nat. Gas Geosci. 6, 245 (2021)
A. Marcinek, A. Möller, J. Guderian, D. Bathen, Adsorption 27, 1149 (2021)
Y. Ma, J. Pan, D. Fu, Y. Wang, Y. Wang, D. Chang, L. Cheng, W. Fu, J. Nat. Gas Eng. 96, 104287 (2021)
M. Pendurthi, V. B. Pelluru, A. Chilakapati, D. Dandotiya, N. D. Banker, J. Therm. Sci. Eng. Appl. 13 (2021).
D. Liu, D. Ren, K. Du, Y. Qi, F. Ye, J. Pet. Sci. Eng. 201, 108397 (2021)
R. Wang, H. Liu, L. Dou, H. Bai, N. Zhang, J. Nat. Gas Eng. 95, 104018 (2021)
W.-S. Sun, M.-J. Yin, W.-H. Zhang, S. Li, N. Wang, Q.-F. An, ACS Sustainable Chem. Eng. 9, 10167 (2021)
S. Li, Y.-J. Sun, Z.-X. Wang, C.-G. Jin, M.-J. Yin, Q.-F. An, Small n/a, 2208177 (2023).
Y. Xu, Z. Lun, X. Zhou, G. Zhang, H. Wang, C. Zhao, H. Zhang, D. Zhang, J. Nat. Gas Eng. 101, 104489 (2022)
B. Wang, X. Liao, W. Tang, S. Li, Q. Jiang, J. Yang, J. Li, R. Li, C. Tian, G. Li, J. Supercrit. Fluids 186, 105610 (2022)
L.M. Cai, K. Surve, J. Yun, A. Zolfaghari, X. Chen, A.K. Bhowmick, R. Krishnamoorti, Ind. Eng. Chem. Res. 61, 16721 (2022)
J. Wu, L. Sun, K. Jessen, T. Tsotsis, Chem. Eng. Sci. 247, 117068 (2022)
R.M. Alloush, K.V. Sharma, M. Piri, J. Mol. Liq. 364, 119894 (2022)
S.-F. Wu, L.-W. Wang, G.-L. An, B. Zhang, Energy Stor. Mater. 54, 822 (2023)
D. Wu, X. Chen, F. Miao, X. Xiao, W. Zhai, Energy Fuels 37, 328 (2023)
M. Kosfeld, B. Westphal, A. Kwade, J. Energy Storage 57, 106174 (2023)
F. Dreisbach, R. Seif, H.W. Lösch, Chem. Eng. Technol. 25, 1060 (2002)
R. Pini, Microporous Mesoporous Mater. 187, 40 (2014)
S. Brandani, E. Mangano, L. Sarkisov, Adsorption 22, 261 (2016)
X. Yang, A. Arami-Niya, J. Lyu, X. Guo, J. Chem. Eng. Data 66, 404 (2020)
J. Toth, Acta Chim. Hung. 69, 311 (1971)
S. Ono and S. Kondo, Molecular theory of surface tension in liquids, in Structure of Liquids/Struktur Der Flüssigkeiten (Springer, 1960), pp. 134–280.
D. Shade, B.W.S. Bout, D.S. Sholl, K.S. Walton, Ind. Eng. Chem. Res. 61, 2367 (2022)
J.U. Keller, N. Iossifova, W. Zimmermann, Adsorpt. Sci. Technol. 23, 685 (2005)
X. Yang, E. Sadeghi Pouya, G. Xiao, M. Richter, E.F. May, Langmuir 39, 13521 (2023)
Y. Wang, M.D. LeVan, J. Chem. Eng. Data 54, 2839 (2009)
S. Cavenati, C.A. Grande, A.E. Rodrigues, J. Chem. Eng. Data 49, 1095 (2004)
S.H. Hyun, R.P. Danner, J. Chem. Eng. Data 27, 196 (1982)
H. Deng, H. Yi, X. Tang, Q. Yu, P. Ning, L. Yang, Chem. Eng. J. 188, 77 (2012)
J.P. Marco-Lozar, J. Juan-Juan, F. Suárez-García, D. Cazorla-Amorós, A. Linares-Solano, Int. J. Hydrog. Energy 37, 2370 (2012)
D. Saha, Z. Wei, S. Deng, Sep. Purif. Technol. 64, 280 (2009)
A.C. Chien, S.S.C. Chuang, Int. J. Hydrog. Energy 36, 6022 (2011)
Z. Xin, J. Bai, Y. Pan, M.J. Zaworotko, Chem. Eur. J. 16, 13049 (2010)
A. Dailly, J.J. Vajo, C.C. Ahn, J. Phys. Chem. B 110, 1099 (2006)
M. Hirscher and B. Panella, J. Alloys Compd. 404–406, 399 (2005)
B. Panella and M. Hirscher, Adv. Mater. 17, 538 (2005)
J.A. Greathouse, T.L. Kinnibrugh, M.D. Allendorf, Ind. Eng. Chem. Res. 48, 3425 (2009)
J.Y. Lee, H. Wu, J. Li, Int. J. Hydrog. Energy 37, 10473 (2012)
Q. Yang, C. Zhong, J. Phys. Chem. B 110, 17776 (2006)
B. Panella, M. Hirscher, H. Pütter, U. Müller, Adv. Funct. Mater. 16, 520 (2006)
W. Cao, Y. Li, L. Wang, S. Liao, J. Phys. Chem. C 115, 13829 (2011)
M. Gallo, D. Glossman-Mitnik, J. Phys. Chem. C 113, 6634 (2009)
A.W. Thornton, K.M. Nairn, J.M. Hill, A.J. Hill, M.R. Hill, J. Am. Chem. Soc. 131, 10662 (2009)
S.J. Yang, J.Y. Choi, H.K. Chae, J.H. Cho, K.S. Nahm, C.R. Park, Chem. Mater. 21, 1893 (2009)
S.J. Yang, J.H. Cho, K.S. Nahm, C.R. Park, Int. J. Hydrog. Energy 35, 13062 (2010)
G. Garberoglio, A.I. Skoulidas, J.K. Johnson, J. Phys. Chem. B 109, 13094 (2005)
Y. Liu, H. Liu, Y. Hu, J. Jiang, J. Phys. Chem. B 113, 12326 (2009)
S.-Y. Lee, S.-J. Park, Int. J. Hydrog. Energy 36, 8381 (2011)
D. Rao, R. Lu, C. Xiao, E. Kan, K. Deng, Chem. Commun. 47, 7698 (2011)
H.W. Langmi, J. Ren, B. North, M. Mathe, D. Bessarabov, Electrochim. Acta 128, 368 (2014)
J. Kim, S. Yeo, J.-D. Jeon, S.-Y. Kwak, Microporous Mesoporous Mater. 202, 8 (2015)
S.S. Han, D.H. Jung, S. Choi, J. Heo, ChemPhysChem 14, 2698 (2013)
P. Ryan, L. J. Broadbelt, R. Q. Snurr, Chem. Commun. 4132 (2008).
H. Frost, R.Q. Snurr, J. Phys. Chem. C 111, 18794 (2007)
A.W. Thornton, S.A. Furman, K.M. Nairn, A.J. Hill, J.M. Hill, M.R. Hill, Microporous Mesoporous Mater. 167, 188 (2013)
D.W. Siderius, L.D. Gelb, Langmuir 25, 1296 (2009)
D.W. Siderius, V.K. Shen, R.D. Johnson III, R.D. van Zee (eds.), NIST/ARPA-E Database of Novel and Emerging Adsorbent Materials (National Institute of Standards and Technology, Gaithersburg, MD, 2020). https://doi.org/10.18434/T43882. Accessed 27 Oct 2023.
H.G.T. Nguyen, L. Espinal, R.D. van Zee, M. Thommes, B. Toman, M.S.L. Hudson, E. Mangano, S. Brandani, D.P. Broom, M.J. Benham, Adsorption 24, 531 (2018)
H.G.T. Nguyen, C.M. Sims, B. Toman, J. Horn, R.D. van Zee, M. Thommes, R. Ahmad, J.F.M. Denayer, G.V. Baron, E. Napolitano, M. Bielewski, E. Mangano, S. Brandani, D.P. Broom, M.J. Benham, A. Dailly, F. Dreisbach, S. Edubilli, S. Gumma, J. Möllmer, M. Lange, M. Tian, T.J. Mays, T. Shigeoka, S. Yamakita, M. Hakuman, Y. Nakada, K. Nakai, J. Hwang, R. Pini, H. Jiang, A.D. Ebner, M.A. Nicholson, J.A. Ritter, J. Farrando-Pérez, C. Cuadrado-Collados, J. Silvestre-Albero, C. Tampaxis, T. Steriotis, D. Řimnáčová, M. Švábová, M. Vorokhta, H. Wang, E. Bovens, N. Heymans, G. De Weireld, Adsorption 26, 1253 (2020)
G. Lipinski, C. Holzammer, M. Petermann, M. Richter, Meas. Sci. Technol. 29, 105501 (2018)
K. Yang, P.R.J. Conolly, L. Liu, X. Yang, N. Robinson, M. Li, M. Mahmoud, A. El-Husseiny, M. Verrall, E.F. May, M.L. Johns, J. Nat. Gas Eng. 108, 104847 (2022)
M.O. McLinden, R. Kleinrahm, W. Wagner, Int. J. Thermophys. 28, 429 (2007)
D. Lozano-Martín, M.E. Mondéjar, J.J. Segovia, C.R. Chamorro, Measurement 151, 107176 (2020)
D.E. Cristancho, I.D. Mantilla, S. Ejaz, K.R. Hall, G.A. Iglesias-Silva, M. Atilhan, Int. J. Thermophys. 31, 698 (2010)
Y. Kano, Y. Kayukawa, K. Fujii, H. Sato, Meas. Sci. Technol. 18, 659 (2007)
G.L. Harris, J.A. Torres, Selected Laboratory and Measurement Practices and Procedures to Support Basic Mass Calibrations (NIST Interagency/Internal Report (NISTIR), National Institute of Standards and Technology, Gaithersburg, MD, 2003)
H.A. Bowman, R.M. Schoonover, C.L. Carroll, J Res Natl Bur Stand A 78A, 13 (1974)
H.A. Bowman, R.M. Schoonover, C.L. Carroll, Metrologia 10, 117 (1974)
F. Xu, M. Song, C. Chen, X. Xu, IEEE Trans. Instrum. Meas. 72, 1 (2023)
Acknowledgment
X.Y. thanks his students for supporting this work through detailed literature review.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work has received no funding.
Author information
Authors and Affiliations
Contributions
Conceptualization: XY, RK, MM, and MR; Data curation: XY and MR; Resources: MR; Visualization: XY and MM; Writing—original draft: XY, RK, and MM; Writing—review and editing: MR.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this paper.
Consent to Participate
The authors consent to participate.
Consent for Publication
All authors read the final version of the manuscript and consent to publish.
Ethical Approval
The work contains no libelous or unlawful statements, does not infringe on the rights of others, or contains material or instructions that might cause harm or injury.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Special Issue in Honor of Professor Roland Span’s 60th Birthday.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, X., Kleinrahm, R., McLinden, M.O. et al. The Magnetic Suspension Balance: 40 Years of Advancing Densimetry and Sorption Science. Int J Thermophys 44, 169 (2023). https://doi.org/10.1007/s10765-023-03269-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-023-03269-0