Abstract
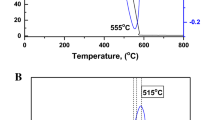
Heteroatoms are known to introduce specific surface functionalities that can enhance the adsorption properties of carbons. Sulfur fixation on bagasse-activated carbon was conducted by a low temperature chemical treatment with sulfuric acid followed by physical activation with CO2 at 900 °C. The effect of sulfur fixation on the surface chemical properties of bagasse-activated carbons were investigated and on their subsequent acid dye removal (CIBA AB80) behavior. Surface chemical development were examined and followed using Fourier transform infrared spectroscopy (FTIR), heteroatom analysis and carbon surface acidity. Functional group stability with thermal treatment was also investigated. The textural properties of the activated carbons were characterized by nitrogen adsorption. Chemical pre-treatment and gasification was able to fix up to 0.2 wt% of sulfur on the activated carbon. Although the sulfur fixed by chemical treatment is low, this method introduced several advantages in comparison to fixation by thermal methods. The chemical method did not interfere with the textural development of the carbon, as found in thermal methods. In addition, the surface chemistry generated by these levels of sulfur groups was sufficient to increase the uptake of acid blue dyes by more than 700% based on adsorption capacities normalized by the surface area of the carbon.
Similar content being viewed by others
References
ASTMA: In: Annual Book of ASTM Standards: Refractories, Carbon and Graphite Products; Activated Carbons. Easton (1996)
Al-Degs, Y., Khraisheh, M.A.M., Allen, S.J., Ahmad, M.N.: Effect of carbon surface chemistry on the removal of reactive dyes from textile effluent. Water Res. 34(3), 927–935 (2000)
Baclioglu, I.A., Arsan, I.: Partial oxidation of reactive dyestuff and synthetic textile dye-bath by the O3 and O3/H2O2 processes. Water Sci. Tech. 43, 221–228 (2001)
Bandosz, T.J., Ania, C.O.: Surface chemistry of activated carbons and its characterization. In: Bandosz, T.J. (ed.) Activated Carbon Surfaces in Environmental Remediation. Academic Press, Amsterdam (2006)
Bansal, R.C., Gupta, U.: Fixation of sulfur by carbon-blacks on treatment with aqueous-solutions of hydrogen-sulfide. Indian J. Technol. 18(3), 131–133 (1980)
Brunauer, S., Emmett, P.H., Teller, E.: Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 60, 309–319 (1938)
Chan, L.S., Cheung, W.H., McKay, G.: Adsorption of acid dyes by bamboo derived activated carbon. Desalination 218(1–3), 304–312 (2008)
Choy, K.K.H., Porter, J.F., McKay, G.: Langmuir isotherm models applied to the multicomponent sorption of acid dyes from effluent onto activated carbon. J. Chem. Eng. Data 45(4), 575–584 (2000)
Corapcioglu, M.O., Huang, C.P.: The surface-acidity and characterization of some commercial activated carbons. Carbon 25(4), 569–578 (1987)
Freundlich, H.: Concerning adsorption in solutions. Z. Physik. Chem. A 57(4), 385–470 (1906)
Horvath, G., Kawazoe, K.: Method for the calculation of effective pore-size distribution in molecular-sieve carbon. J. Chem. Eng. Jpn. 16(6), 470–475 (1983)
Juang, R.S., Tseng, R.L., Wu, F.C.: Use of chitin and chitosan in lobster shell wastes for colour removal from aqueous solutions. J. Environ. Sci. Health, Part A Environ. Sci. Eng. Toxic Hazard. Subst. Control 31(2), 325–338 (1996)
Khalil, L.B., Girgis, B.S.: Column removal of some dyestuffs by activated carbons derived from apricot stone shells. Adsorpt. Sci. Technol. 16(5), 405–414 (1998)
Krishnan, K.A., Anirudhan, T.S.: Removal of mercury(II) from aqueous solutions and chlor-alkali industry effluent by steam activated and sulphurised activated carbons prepared from bagasse pith: kinetics and equilibrium studies. J. Hazard. Mater. 92(2), 161–183 (2002)
Langmuir, I.: The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 40, 1361–1403 (1918)
Puri, B.R., Hazra, R.S.: Carbon-sulphur surface complexes on charcoal. Carbon 9(2), 123–134 (1971)
Puri, B.R., Kaistha, B., Hazra, R.S.: Studies in formation and properties of carbonsulphur surface complexes. 4. Oxidation of combined sulphur by different oxidising solutions. J. Indian. Chem. Soc. 45(11), 1001–1005 (1968)
Reife, A.: In: Kroschwitc, M. (ed.) Environmental Chemistry of Dyes and Pigment. Wiley, New York (1993)
Sykes, K.W., White, P.: The reactions of carbon with sulphur compounds. 4. Adsorption of gaseous sulphur and carbon disulphide by charcoal. Trans. Faraday. Soc. 52(5), 660–671 (1956)
Syna, N., Valix, M.: Modelling of gold (I) cyanide adsorption based on the properties of activated bagasse. Miner. Eng. 16(5), 421–427 (2003)
Valix, M., Cheung, W.H., McKay, G.: Preparation of activated carbon using low temperature carbonisation and physical activation of high ash raw bagasse for acid dye adsorption. Chemosphere 56(5), 493–501 (2004)
Valix, M., Cheung, W.H., McKay, G.: Roles of the textural and surface chemical properties of activated carbon in the adsorption of acid blue dye. Langmuir 22(10), 4574–4582 (2006)
Wu, F.C., Tseng, R.L., Juang, R.S.: Preparation of activated carbons from bamboo and their adsorption abilities for dyes and phenol. J. Environ. Sci. Health, Part A Toxic/Hazard. Subst. Environ. Eng. 34(9), 1753–1775 (1999a)
Wu, F.C., Tseng, R.L., Juang, R.S.: Pore structure and adsorption performance of the activated carbons prepared from plum kernels. J. Hazard. Mater. 69(3), 287–302 (1999b)
Yamaki, J., Takatsuji, H., Kawamura, T., Egashira, M.: Solid State Ionics 148(3–4), 241–245 (2002)
Zhang, K., Cheung, W.H., Valix, M.: Roles of physical and chemical properties of activated carbon in the adsorption of lead ions. Chemosphere 60(8), 1129–1140 (2005)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Valix, M., Cheung, W.H. & McKay, G. Sulfur fixation on bagasse activated carbon by chemical treatment and its effect on acid dye adsorption. Adsorption 15, 453–459 (2009). https://doi.org/10.1007/s10450-009-9194-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-009-9194-5