Abstract
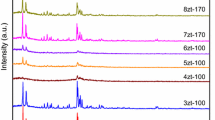
ZSM-5 zeolites with SiO2/Al2O3 molar ratio of 24 were treated in 0.05 M aqueous sodium hydroxide solution at 325 K in different periods. The samples were characterized by means of nitrogen adsorption at 77 K, field emission scanning electron microscopy, X-ray diffractometry, and Fourier transform infrared spectroscopy. Analysis of the experimental results showed that the alkaline treatment periods have influence on the developments and structures of mesopores in the alkaline-treated ZSM-5 zeolites. Alkaline treatment initially develops mesopores mainly from the boundary portion of MFI zeolites to the bulk, while prolonged treatment destroys the mesopores, and an optimum mesoporosity is obtained by the treatment for 1.5 h. On the other hand, crystallinities and short-range order in alkaline treated zeolites have remained virtually unchanged according to the examination from X-ray diffractometry and Fourier transform infrared spectroscopy.
Similar content being viewed by others
References
Argauer, R.J. and G.R. Landolt, “Crystalline Zeolite ZSM-5 and Method of Preparing the Same,” U.S. Patent 3702886 (1972).
Barret, P., L.G. Joyner, and P.P. Halenda, “The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms,” J. Am. Chem. Soc., 73, 373–380 (1951).
Breck D.W., Zeolite Molecular Sieves, pp. 64–67, Robert E. Krieger Publishing Company, Malabar, FL, 1974.
Brunauer S., P.H. Emmett, and E. Teller, “Adsorption of Gases in Multimolecular Layers,” J. Am. Chem. Soc., 60, 309–319 (1938).
Cartlidge, S., H.-U. Nissen, and R. Wessicken, “Ternary Mesoporous Structure of Ultrastable Zeolite CSZ-1,” Zeolites, 9, 346–349 (1989).
Chen N.Y. and T.F. Degnan, “Industrial Catalytic Applications of Zeolites,” Chem. Eng. Prog., 84, 32–41 (1988).
Choi-Feng, C., J.B. Hall, B.J. Huggins, and R.A. Begerlein, “Electron Microscope Investigation of Mesopore Formation and Aluminum Migration in USY Catalysts,” J. Catal., 140, 395–405 (1993).
Christensen, C.H., I. Schmidt, A. Carlsson, K. Johannsen, and K. Herbst, “Crystals in Crystals-Nanocrystals within Mesoporous Zeolite Single Crystals,” J. Am. Chem. Soc., 127, 8098–8102 (2005).
Cizmek, B., B. Suboti, R. Aiello, F. Crea, A. Nastro, and C. Tuoto, “Dissolution of High-silica Zeolites in Alkaline Solutions I. Dissolution of Silicalite-1 and ZSM-5 with Different Aluminum Content,” Microporous Materials, 4, 159–168 (1995).
Cizmek, B., B. Suboti, I. Smit, A. Tonejc, R. Aiello, F. Crea, and A. Nastro, “Dissolution of High-silica Zeolites in Alkaline Solutions II. Dissolution of ‘Activated’ Silicalite-1 and ZSM-5 with Different Aluminum Content,” Microporous Mater., 8, 159–169 (1997).
Corma, A., “From Microporous to Mesoporous Molecular Sieve Materials and Their Use in Catalysis,” Chem. Rev., 97, 2373–2420 (1997).
Corma, A., M.J. Diaz-Cabanas, J. Martinez-Triguero, F. Rey, and J. Rius, “A Large-cavity Zeolite with Wide Pore Windows and Potential as an Oil Refining Catalyst,” Nature, 418, 514–517 (2002).
Corma, A., “State of the Art and Future Challenges of Zeolites as Catalysts,” J. Catal., 216, 298–312 (2003).
Davis, M.E., “Ordered Porous Materials for Emerging Applications,” Nature, 417, 813–821 (2002).
Ghose S. and B. Mattiasson, “Protein Adsorption to Hydrophobic Zeolite Y: Salt effects and Application to Protein Fractionation,” Biotechnol. Appl. Biochem, 18, 311–320 (1993).
Gregg, S.J. and K.S.W. Sing, Adsorption, Surface Area and Porosity, pp 111–194, 2nd edition, Academic Press, London, 1982.
Groen, J.C., J.A. Moulijn, and J. Pérez-Ramírez, “Decoupling Mesoporosity Formation and Acidity Modification in ZSM-5 Zeolites by Sequential Desilication–dealumination,” Microporous Mesoporous Mater., 87, 153–161 (2005a).
Groen, J.C., J.C. Jansen, J.A. Moulijn, and J. Pérez–Ramírez, “Optiomal Aluminum-Assised Mesoporosity Development in MFI Zeolites by Desilication,” J. Phys. Chem. B, 108, 13062–13065 (2004).
Groen, J.C., J.Pérez-Ramírez, and L.A.A. Peffer, “Formation of Uniform Mesopores in ZSM-5 Zeolite upon AlKaline Post-treatment,” Chem. Lett., 94–94 (2002).
Groen, J.C., L.A.A. Peffer, and J. Pérez-Ramírez, “Pore Size Determination in Modified Micro- and Mesoporous Materials. Pitfalls and Limitations in Gas Adsorption Data Analysis,” Microporous Mesoporous Mater., 60, 1–17 (2003).
Groen, J.C., L.A.A. Peffer, J.A. Moulijn, and J. Pérez-Ramírez, “Mechanism of Hierachical Porosity Development in MFI Zeolites by Desilication: The Role of Aluminium as a Pore-Directing Agent,” Chem. Eur. J., 11, 4983–4994 (2005b).
Groen, J.C., S. Brouwer, L.A.A. Peffer, and J. Pérez-Ramírez, “Application of Mercury Intrusion Porosimetry for Characterization of Combined Micro- and Mesoporous Zeolites,” Part. Part. Syst. Charact, 23, 101–106 (2006).
Groen, J.C., T. Bach, U. Ziese, A.M. Paulaime-van Donk, K.P. de Jong, J.A. Moulijn, and J. Pérez-Ramírez, “Creation of Hollow Zeolite Architectures by Controlled Desillication of Al-Zoned ZSM-5 Crystals,” J. Am. Chem. Soc., 127, 10792–10793 (2005c).
Hartman, M., “Hierarchical Zeolites: A Proven Strategy to Combine Shape Selectivity with Efficient Mass Transport,” Angew. Chem, Int. Ed., 43, 5880–5882 (2004).
Herrmann, C., J. Haas, and F. Fetting, “Effect of the Crystal Size on the Activity of ZSM-5 Catalysts in Various Reactions,” Appl. Catal., 35, 299–310 (1987).
Jacobsen, C.J.H., C. Madsen, J. Houzvicka, I. Schmidt, and A. Carlsson, “Mesoporous Zeolite Single Crystals,” J. Am. Chem. Soc., 122, 7116–7117 (2000).
Jansen, J.C., F.J. van der Gaag, and H. van Bekkum, “Identification of ZSM-type and Other 5-ring Containing Zeolites by i.r. Spectroscopy,” Zeolites, 369–372 (1984).
Janssen, A.H., I. Schmidt, C.J.H. Jacobsen, A.J. Koster, and K.P. de Jong, “Exploratory Study of Mesopore Templating with Carbon During Zeolite Synthesis,” Microporous Mesoporous Mater., 65, 59–75 (2003).
Kärger J. and D.M. Ruthven, Diffusion in Zeolites and Other Microporous Materials, pp. 375–426, Wiley, New York, 1992.
Lynch, J., F. Raatz, and P. Dufresne, “Characterization of the Textural Properties of Dealuminated HY Forms,” Zeolites, 7, 333–340 (1987).
Ogura, M., S. Shinomiya, J. Tateno, Y. Nara, E. Kikuchi, and M. Matsukata, “Formation of Uniform Mesopores in ZSM-5 Zeolite through Treatment in Alkaline Solution,” Chem. Lett., 882–883 (2000).
Ogura, M., S. Shinomiya, J. Tateno, Y. Nara, M. Nomura, E. Kikuchi, and M. Matsukata, “Alkali-treatment Technique – New Method for Modification of Structural and Acid-catalytic Properties of ZSM-5 Zeolites,” Appl. Catal. A, 219, 33–43 (2001).
Patzelová, V. and N.I. Jaeger, “Texture of Deep Bed Treated Y Zeolites,” Zeolites, 7, 240–242 (1987).
Perez-Ramirez J., F. Kapteijn, J.C. Groen, A. Domenech, G. Mul, and J.A. Moulijn, “Steam-activated FeMFI Zeolites. Evolution of Iron Species and Activity in Direct N2O Decomposition,” J. Catal., 214, 33–45 (2003).
Pires J., A. Carvalho, and M.B. de Carvaho, “Adsorption of Volatile Organic Compounds in Y Zeolites and Pillared Clays,” Micropor. Mesopor. Mater., 43, 277–287 (2001).
Richter M., H. Berndt, R. Eckelt, M. Schneider, and R. Fricke, “Zeolite-mediated Removal of NOx by NH3 from Exhaust Streams at Low Temperatures,” Catalysis Today, 54, 531–545 (1999).
Saito, A. and H.C. Foley, “Argon Porosimetry of Selected Molecular Sieves: Experiments and Examination of the Adapted Horvath-Kawazoe Model,” Microporous Mater., 3, 531–542 (1995).
Saito, A. and H.C. Foley, “Curvature and Parametric Sensitivity in Models for Adsorption in Micropores,” AIChE J., 37, 429–436 (1991).
Sakthivel, A., S. Huang, W. Chen, Z. Lan, K. Chen, T. Kim, R. Ryoo, A.S.T. Chiang, and S. Liu, “Replication of Mesoporous Aluminosilicate Molecular Sieves (RMMs) with Zeolite Framework from Mesoporous Carbons (CMKs),” Chem. Mater., 16, 3168–3175 (2004).
Schmidt, I., A. Boisen, E. Gustavsson, K. Stahl, S. Pehrson, S. Dahl, A. Carlsson, and C.J.H. Jacobsen, “Carbon Nanotube Templated Growth of Mesoporous Zeolite Single Crystals,” Chem. Mater., 13, 4416–4418 (2001).
Scholle, K.F.M.G.J., W.S. Veeman, P. Frenken, and G.P.M. van der Velden, “Characterization of Intermediate TPA-ZSM-5 Type Structures During Crystallization,” Appl. Catal., 17, 233–259 (1985).
Smith, J.V., “Topochemistry of Zeolites and Related Materials. 1. Topology and Geometry,” Chem. Rev., 88, 149–182 (1988).
Suzuki, T. and T. Okuhara, “Change in Pore Structure of MFI Zeolite by Treatment with NaOH Aqueous Solution,” Micropor. Mesopor. Mater., 43, 83–89 (2001).
Tao, Y., H.Kanoh, and K. Kaneko, “ZSM-5 Monolith of Uniform Mesoporous Channels,” J. Am. Chem. Soc., 125, 6044–6045 (2003).
Tao, Y., H. Kanoh, J.C. Groen, and K. Kaneko, “Characterization of Alkaline Post-treated ZSM-5 Zeolites by Low Temperature Nitrogen adsorption,” Stud. Surf. Sci. Catal., in press.
Tao, Y.H., Kanoh, L. Abrams, and K. Kaneko, “Mesopore-Modified Zeolites: Preparation, Characterization, and Appliations,” Chem. Rev., 106, 896–910 (2006).
van Donk, S., A. Broersma, O.L.J. Gijzeman, J.A. van Bokhoven, J.H. Bitter, and K.P. de Jong, “Combined Diffusion, Adsorption, and Reaction Studies of n-Hexane Hydroisomerization over Pt/H–Mordenite in an Oscillating Microbalance,” J. Catal., 204, 272–280 (2001).
van Donk, S., A.H. Janssen, J.H. Bitter, and K.P. de Jong, “Generation, Characterization, and Impact of Mesopores in Zeolite Catalysts,” Catal. Rev., 45, 297–319 (2003).
Wu, E.L., S.L. Lawton, D.H. Olson, A.C., Jr. Rohrman, and G.T. Kokotailo, “ZSM-5-Type Materials. Factors Affecting Crystal Symmetry,” J. Phys. Chem., 83, 2777–2781 (1979).
Yang, Z., Y. Xia, and R. Mokaya, “Zeolite ZSM-5 with Unique Supermicropores Synthesized Using Mesoporous Carbon as a Template,” Adv. Mater., 16, 727–732 (2004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tao, Y., Kanoh, H. & Kaneko, K. Developments and structures of mesopores in alkaline-treated ZSM-5 zeolites. Adsorption 12, 309–316 (2006). https://doi.org/10.1007/s10450-006-0561-1
Issue Date:
DOI: https://doi.org/10.1007/s10450-006-0561-1