Abstract
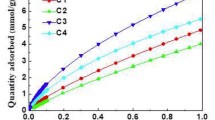
The kinetics of the adsorption of CO2 at 296 K in the micropores of sol-gel derived, monolithic carbons indicates two different types of micropores. While the micropore volume as well as the micropore width of both species turns out to be very similar, their accessibility is significantly different. To study the adsorption kinetics in more detail a set of sol-gel derived hard carbons was prepared with average macropore sizes ranging from about 50 nm to 5 micron at a total porosity of about 85%. To characterize the morphology of the samples small-angle X-ray scattering and N2 sorption at 77 K were applied. Evaluation of the structural characteristics and the adsorption kinetics of the carbons investigated reveals that the two different types of micropores are homogeneously spread throughout the carbon backbone. The adsorption kinetics in the readily accessible type of micropores is dominated by the gas phase transport through the macropores combined with transport along the inner surface. In contrast, the access to the second category of micropores is highly restricted even at 296 K; this is reflected in equilibration times of about 500 s. The characteristics of the slow adsorption component is almost independent of the macroscopic size and the morphology of the specimen, suggesting that the slow kinetics is controlled by the local accessibility of the micropores. Surprisingly, the equilibrium data of the two adsorption components as a function of the relative pressure reveal that the micropores that are only slowly filled are actually characterized by a lower (Dubinin-Raduskevich) energy and thus a larger micropore width than the ones that are readily filled. This can be interpreted in terms of micropores with a very narrow entrance (restricted diffusion) or a widening of the micropores due to swelling of the carbon upon CO2 adsorption.
Similar content being viewed by others
References
Alcaniz-Monge, C. et al., Carbon, 40, 541 (2002).
Barbieri, O. et al., Journal of Non-Crystalline Solids, 285, 109–115 (2001).
Fricke, J. and R. Petricevic, Carbon Aerogels, Handbook of Porous Solids, F. Schüth, K. Sing, and J. Weitkamp (Eds.), Wiley-VCH, Weinheim, 2002, pp. 2037–2062.
Gondy, D. and P. Ehrburger, Carbon, 35, 1745 (1997).
5Nakashima, M. et al., Carbon, 33, 1301 (1995).
6Przepiorski, J. et al., Applied Surface Science, 196, 296 (2002).
Reichenauer, G. et al., “Monitoring Fast Pressure Changes in Gas Transport and Sorption Analysis,”Studies in Surface and Catalysis Vol. 144, F. Rodriguez-Reinoso, B. McEnaney, J. Rouquerol, and K. Unger (Eds.), Elsevier Science B.V.2002, p. 443.
Rodriguez, C.F. and M.J. Lemos de Sousa, International Journal of Coal Geology, 48, 245 (2002).
Saliger, R. et al., “Evolution of Microporosity upon CO2-Activation of Carbon Aerogels,”Characterization of Porous Solids V, Unger K.K. et al. (Eds.),2000, p. 381.
Stöckli, et al., Carbon, 28, 907(1990).
Stumpf, C. et al., Journal of Non-Crystalline Solids, 145, 180(1992).
Yang, R.T., Gas Separation by Adsorption Processes, Imperial College Press 1997, pp. 5, 269 et seq.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reichenauer, G. Micropore Adsorption Dynamics in Synthetic Hard Carbons. Adsorption 11 (Suppl 1), 467–471 (2005). https://doi.org/10.1007/s10450-005-5969-5
Issue Date:
DOI: https://doi.org/10.1007/s10450-005-5969-5