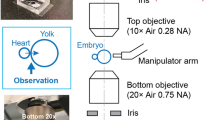
Abstract
Cardiac morphogenesis requires an intricate orchestration of mechanical stress to sculpt the heart as it transitions from a straight tube to a multichambered adult heart. Mechanical properties are fundamental to this process, involved in a complex interplay with function, morphology, and mechanotransduction. In the current work, we propose a pressurization technique applied to the zebrafish atrium to quantify mechanical properties of the myocardium under passive tension. By further measuring deformation, we obtain a pressure-stretch relationship that is used to identify constitutive models of the zebrafish embryonic cardiac tissue. Two-dimensional results are compared with a three-dimensional finite element analysis based on reconstructed embryonic heart geometry. Through these steps, we found that the myocardium of zebrafish results in a stiffness on the order of 10 kPa immediately after the looping stage of development. This work enables the ability to determine how these properties change under normal and pathological heart development.








Similar content being viewed by others
References
Azeloglu, E. U., and K. D. Costa. Cross-bridge cycling gives rise to spatiotemporal heterogeneity of dynamic subcellular mechanics in cardiac myocytes probed with atomic force microscopy. Am. J. Physiol-Heart Circ. Physiol. 298:H853–H860, 2010.
Bark, D., B. Johnson, D. Garrity, and L. Dasi. Valveless pumping mechanics of the embryonic heart during cardiac looping: pressure and flow through micro-PIV. J. Biomech. 50:50–55, 2017.
Bartman, T., E. C. Walsh, K. K. Wen, M. McKane, J. H. Ren, J. Alexander, P. A. Rubenstein, and D. Y. R. Stainier. Early myocardial function affects endocardial cushion development in zebrafish. PLoS Biol. 2:673–681, 2004.
Costa, K. D., A. J. Sim, and F. C. P. Yin. Non-Hertzian approach to analyzing mechanical properties of endothelial cells probed by atomic force microscopy. J. Biomech. Eng. 128:176–184, 2005.
Delfino, A., N. Stergiopulos, J. Moore, Jr, and J.-J. Meister. Residual strain effects on the stress field in a thick wall finite element model of the human carotid bifurcation. J. Biomech. 30:777–786, 1997.
Demiray, H., and R. P. Vito. A layered cylindrical shell model for an aorta. Int. J. Eng. Sci. 29:47–54, 1991.
Dokos, S., B. H. Smaill, A. A. Young, and I. J. LeGrice. Shear properties of passive ventricular myocardium. Am. J. Physiol-Heart Circ. Physiol. 283:H2650–H2659, 2002.
Ebert, A., G. Hume, K. Warren, N. Cook, C. Burns, M. Mohideen, G. Siegal, D. Yelon, M. Fishman, and D. Garrity. Calcium extrusion is critical for cardiac morphogenesis and rhythm in embryonic zebrafish hearts. Proc. Natl. Acad. Sci. 102:17705–17710, 2005.
Engler, A. J., C. Carag-Krieger, C. P. Johnson, M. Raab, H.-Y. Tang, D. W. Speicher, J. W. Sanger, J. M. Sanger, and D. E. Discher. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J. Cell Sci. 121:3794–3802, 2008.
Glickman N. S., D. Yelon. Cardiac development in zebrafish: coordination of form and function. In: Seminars in Cell & Developmental Biology Elsevier, pp. 507–513, 2002.
Haack, T., and S. Abdelilah-Seyfried. The force within: endocardial development, mechanotransduction and signalling during cardiac morphogenesis. Development 143:373–386, 2016.
Hamburger, V., and H. L. Hamilton. A series of normal stages in the development of the chick embryo. J. Morphol. 88:49–92, 1951.
Herrmann, C., J. Wray, F. Travers, and T. Barman. Effect of 2,3-butanedione monoxime on myosin and myofibrillar ATPases. An example of an uncompetitive inhibitor. Biochemistry 31:12227–12232, 1992.
Holzapfel, A. G. Nonlinear Solid Mechanics. New York: Wiley, 2000.
Hove, J. R., R. W. Koster, A. S. Forouhar, G. Acevedo-Bolton, S. E. Fraser, and M. Gharib. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature 421:172–177, 2003.
Hu, N., and E. Clark. Hemodynamics of the stage 12 to stage 29 chick embryo. Circ. Res. 65:1665–1670, 1989.
Hu, N., and B. B. Keller. Relationship of simultaneous atrial and ventricular pressures in stage 16-27 chick embryos. Am. J. Physiol-Heart Circ. Physiol. 269:H1359–H1362, 1995.
Hu, N., H. J. Yost, and E. B. Clark. Cardiac morphology and blood pressure in the adult zebrafish. Anatom. Record 264:1–12, 2001.
Humphrey, J. D. Cardiovascular Solid Mechanics: Cells, Tissues, and Organs. New York: Springer, 2013.
Ingber, D. E., and I. Tensegrity. Cell structure and hierarchical systems biology. J. Cell Sci. 116:1157–1173, 2003.
Janmey, P. A., and C. A. McCulloch. Cell mechanics: integrating cell responses to mechanical stimuli. Annu. Rev. Biomed. Eng. 9:1–34, 2007.
Johnson, B., D. Bark, Jr, I. Van Herck, D. Garrity, and L. P. Dasi. Altered mechanical state in the embryonic heart results in time-dependent decreases in cardiac function. Biomech. Model. Mechanobiol. 521:1–11, 2015.
Kuznetsova, T. G., M. N. Starodubtseva, N. I. Yegorenkov, S. A. Chizhik, and R. I. Zhdanov. Atomic force microscopy probing of cell elasticity. Micron 38:824–833, 2007.
Lammerding, J., R. D. Kamm, and R. T. Lee. Mechanotransduction in cardiac myocytes. Ann. N. Y. Acad. Sci. 1015:53–70, 2004.
Majkut, S., T. Idema, J. Swift, C. Krieger, A. Liu, and D. E. Discher. Heart-specific stiffening in early embryos parallels matrix and myosin expression to optimize beating. Curr. Biol. 23:2434–2439, 2013.
Makarenko, I., C. Opitz, M. Leake, C. Neagoe, M. Kulke, J. Gwathmey, F. Del Monte, R. Hajjar, and W. Linke. Passive stiffness changes caused by upregulation of compliant titin isoforms in human dilated cardiomyopathy hearts. Circ. Res. 95:708–716, 2004.
Männer, J. Cardiac looping in the chick embryo: a morphological review with special reference to terminological and biomechanical aspects of the looping process. Anatom. Record 259:248–262, 2000.
McCain, M. L., and K. K. Parker. Mechanotransduction: the role of mechanical stress, myocyte shape, and cytoskeletal architecture on cardiac function. Pflügers Arch. Eur. J. Physiol. 462:89, 2011.
Press, C. S. H. L. Danieau’s Solution (30×). New York: Cold Spring Harbor Protocols, 2011.
Samarel, A. M. Costameres, focal adhesions, and cardiomyocyte mechanotransduction. Am. J. Physioly-Heart Circ. Physiol. 289:H2291–H2301, 2005.
Schneider, C. A., W. S. Rasband, and K. W. Eliceiri. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9:671, 2012.
Schroder, E. A., K. Tobita, J. P. Tinney, J. K. Foldes, and B. B. Keller. Microtubule involvement in the adaptation to altered mechanical load in developing chick myocardium. Circ. Res. 91:353–359, 2002.
Sedmera, D., T. Pexieder, V. Rychterova, N. Hu, and E. B. Clark. Remodeling of chick embryonic ventricular myoarchitecture under experimentally changed loading conditions. Anatom. Record 254:238–252, 1999.
Shi, Y., J. Yao, G. Xu, and L. A. Taber. Bending of the looping heart: differential growth revisited. J. Biomech. Eng. 136:081002, 2014.
Stainier, D., B. M. Weinstein, H. R. Detrich, L. I. Zon, and M. C. Fishman. Cloche, an early acting zebrafish gene, is required by both the endothelial and hematopoietic lineages. Development 121:3141–3150, 1995.
Tobita, K., E. A. Schroder, J. P. Tinney, J. B. Garrison, and B. B. Keller. Regional passive ventricular stress-strain relations during development of altered loads in the chick embryo. Am. J. Physiol-Heart Circ. Physiol. 282:H2386–H2396, 2002.
Tracqui, P., and J. Ohayon. Transmission of mechanical stresses within the cytoskeleton of adherent cells: a theoretical analysis based on a multi-component cell model. Acta. Biotheor. 52:323–341, 2004.
Wang, T.-W., and M. Spector. Development of hyaluronic acid-based scaffolds for brain tissue engineering. Acta Biomater. 5:2371–2384, 2009.
Weinstein, B. M., D. L. Stemple, W. Driever, and M. C. Fishman. Gridlock, a localized heritable vascular patterning defect in the zebrafish. Nat. Med. 1:1143–1147, 1995.
Westerfield, M. The Zebrafish Book. Eugene, OR: University of Oregon Press, 1995.
Yao, J., V. D. Varner, L. L. Brilli, J. M. Young, L. A. Taber, and R. Perucchio. Viscoelastic material properties of the myocardium and cardiac jelly in the looping chick heart. J. Biomech. Eng. 134:024502, 2012.
Yeoh, O. H. Some forms of the strain energy function for rubber. Rubber Chem. Technol. 66:754–771, 1993.
Zamir, E. A., V. Srinivasan, R. Perucchio, and L. A. Taber. Mechanical asymmetry in the embryonic chick heart during looping. Ann. Biomed. Eng. 31:1327–1336, 2003.
Zamir, E. A., and L. A. Taber. Material properties and residual stress in the stage 12 chick heart during cardiac looping. J. Biomech. Eng. 126:823–830, 2004.
Zamir, E. A., and L. A. Taber. On the effects of residual stress in microindentation tests of soft tissue structures. J. Biomech. Eng. 126:276–283, 2004.
Zhang, R.-Z., A. A. Gashev, D. C. Zawieja, and M. J. Davis. Length-tension relationships of small arteries, veins, and lymphatics from the rat mesenteric microcirculation. Am J. Physiol-Heart Circ Physiol. 292:H1943–H1952, 2007.
Acknowledgments
We would like to thank Steven Pagano and Mitchell Page for their help with the FEA.
Funding
This work was supported by the American Heart Association [Grant Number 17GRNT33460256].
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Arash Kheradvar oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (AVI 716 kb)
Rights and permissions
About this article
Cite this article
Gendernalik, A., Zebhi, B., Ahuja, N. et al. In Vivo Pressurization of the Zebrafish Embryonic Heart as a Tool to Characterize Tissue Properties During Development. Ann Biomed Eng 49, 834–845 (2021). https://doi.org/10.1007/s10439-020-02619-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-020-02619-5