Abstract
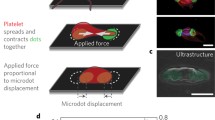
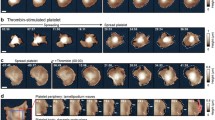
Platelets, essential for hemostasis, are easily activated via biochemical and mechanical stimuli. Cell stiffness is a vital parameter modulating the mechano-transduction of exogenous mechanical stimuli. While methods exist to measure cell stiffness, no ready method exists for measuring platelet stiffness that is both minimally-contacting, imparting minimal exogenous force and non-activating. We developed a minimal-contact methodology capable of trapping and measuring the stiffness of individual platelets utilizing dielectrophoresis (DEP)-mediated electrodeformation. Parametric studies demonstrate a non-uniform electric field in the MHz frequency range (0.2–20 MHz) is required for generating effective DEP forces on platelets, suspended in isotonic buffer with conductivity ~100–200 μS/cm. A nano-Newton DEP force (0.125–4.5 nN) was demonstrated to be essential for platelet electrodeformation, which could be generated with an electric field with strength of 1.5–9 V/μm. Young’s moduli of platelets were calculated using a Maxwell stress tensor model and stress-deformation relationship. Platelet stiffness was determined to be in the range of 3.5 ± 1.4 and 8.5 ± 1.5 kPa for resting and 0.4% paraformaldehyde-treated cells, respectively. The developed methodology fills a gap in approaches of measuring individual platelet stiffness, free of inadvertent platelet activation, which will facilitate further studies of mechanisms involved in mechanically-mediated platelet activation.






Similar content being viewed by others
References
Avrahami, I. and M. Gharib. Effects of membrane stiffening on focal-adhesion bonding under steady and unsteady conditions. In: IEEE Bio Micro and Nanosystems Conference, 2006. BMN’06 2006.
Bakewell, D. J., N. Vergara-Irigaray, and D. Holmes. Dielectrophoresis of Biomolecules. JSM Nanotechnol. Nanomed. 1(1):1003, 2013.
Berger, G., D. W. Hartwell, and D. D. Wagner. P-Selectin and platelet clearance. Blood 92(11):4446–4452, 1998.
Berman, C. L., et al. A platelet alpha granule membrane-protein that is associated with the plasma-membrane after activation—characterization and subcellular-localization of platelet activation-dependent granule-external membrane-protein. J. Clin. Invest. 78(1):130–137, 1986.
Bluestein, D., et al. Device thrombogenicity emulation: A novel methodology for optimizing the thromboresistance of cardiovascular devices (vol 46, pg 334, 2012). J. Biomech. 46(7):1413, 2013.
Chen, J., et al. Electrodeformation for single cell mechanical characterization. J. Micromech. Microeng. 21(5):054012, 2011.
Chen, J., et al. Electrodeformation for Single Cell Mechanical Characterization. 2011 IEEE 24th International Conference on Micro Electro Mechanical Systems (Mems), pp. 1119-1122, 2011.
Cheng, Q., et al. PDMS elastic micropost arrays for studying vascular smooth muscle cells. Sens. Actuators B-Chem. 188:1055–1063, 2013.
Farndale, R. W. Collagen-induced platelet activation. Blood Cells Mol. Dis. 36(2):162–165, 2006.
Ferry, J. D. Viscoelastic Properties of Polymers. New York: Wiley, p. 482, 1961.
Gao, J., et al. Hybrid electrokinetic manipulation in high-conductivity media. Lab Chip 11(10):1770–1775, 2011.
Guck, J., et al. Optical deformability as an inherent cell marker for testing malignant transformation and metastatic competence. Biophys. J. 88(5):3689–3698, 2005.
Guo, Q., S. Park, and H. S. Ma. Microfluidic micropipette aspiration for measuring the deformability of single cells. Lab Chip 12(15):2687–2695, 2012.
Haga, J. H., et al. Quantification of the passive mechanical properties of the resting platelet. Ann. Biomed. Eng. 26(2):268–277, 1998.
Haghi, M., D. Traini, L. G. Wood, B. Oliver, P. M. Young, and W. Chrzanowski. A “soft spot” for drug transport: modulation of cell stiffness using fatty acids and its impact on drug transport in lung model. J. Mater. Chem. B 3:2583–2589, 2015.
Hartwig, J. H. Mechanisms of actin rearrangements mediating platelet activation. J. Cell Biol. 118(6):1421–1442, 1992.
Hou, H. W., et al. Deformability based cell margination - A simple microfluidic design for malarial infected red blood cell filtration. 6th World Congress of. Biomechanics 31:1671–1674, 2010.
Hsulin, S. C., et al. a platelet membrane-protein expressed during platelet activation and secretion—studies using a monoclonal-antibody specific for thrombin-activated platelets. J. Biol. Chem. 259(14):9121–9126, 1984.
Hu, X. Y., et al. Marker-specific sorting of rare cells using dielectrophoresis. Proc. Natl. Acad. Sci. USA 102(44):15757–15761, 2005.
Jesty, J., and D. Bluestein. Acetylated prothrombin as a substrate in the measurement of the procoagulant activity of platelets: elimination of the feedback activation of platelets by thrombin. Anal. Biochem. 272(1):64–70, 1999.
Kapoor, J. R. Platelet activation and atherothrombosis. New Engl. J. Med. 358(15):1638, 2008.
Koay, E. J., A. C. Shieh, and K. A. Athanasiou. Creep indentation of single cells. J. Biomech. Eng.-Trans. ASME 125(3):334–341, 2003.
Kroll, M. H., et al. Platelets and shear stress. Blood 88(5):1525–1541, 1996.
Kuwahara, M., et al. Platelet shape changes and adhesion under high shear flow. Arterioscler. Thromb. Vasc. Biol. 22(2):329–334, 2002.
Lee, S. W., et al. Development of microelectrode arrays for artificial retinal implants using liquid crystal polymers. Invest. Ophthalmol. Vis. Sci. 50(12):5859–5866, 2009.
Leung, S. L., et al. Gold nano-particle-based thermal sensors fabricated using microspotting and DEP techniques. Sens. Actuators A-Phys. 178:32–39, 2012.
Lim, C. T., E. H. Zhou, and S. T. Quek. Mechanical models for living cells—A review. J. Biomech. 39(2):195–216, 2006.
Lincoln, B., et al. Deformability-based flow cytometry. Cytometry Part A 59A(2):203–209, 2004.
Lord, M. S., et al. The modulation of platelet adhesion and activation by chitosan through plasma and extracellular matrix proteins. Biomaterials 32(28):6655–6662, 2011.
Lu, H., et al. Microfluidic shear devices for quantitative analysis of cell adhesion. Anal. Chem. 76(18):5257–5264, 2004.
MacQueen, L. A., M. D. Buschmann, and M. R. Wertheimer. Mechanical properties of mammalian cells in suspension measured by electro-deformation. J. Micromech. Microeng. 20(6):065007, 2010.
Martinez, E. J., Y. Lanir, and S. Einav. Effects of contact-induced membrane stiffening on platelet adhesion. Biomech. Model. Mechanobiol. 2(3):157–167, 2004.
Maugis, D., and M. Barquins. Fracture mechanics and adherence of viscoelastic bodies. J. Phys. D-Appl. Phys. 11(14):1989–2023, 1978.
Morgan, H., M. P. Hughes, and N. G. Green. Separation of submicron bioparticles by dielectrophoresis. Biophys. J. 77(1):516–525, 1999.
Needham, D., and R. M. Hochmuth. Rapid flow of passive neutrophils into a 4 Mu-M pipette and measurement of cytoplasmic viscosity. J. Biomech. Eng.-Trans. ASME 112(3):269–276, 1990.
Neuman, K. C., and A. Nagy. Single-molecule force spectroscopy: optical tweezers, magnetic tweezers and atomic force microscopy. Nat. Methods 5(6):491–505, 2008.
Nobili, M., et al. Platelet activation due to hemodynamic shear stresses: Damage accumulation model and comparison to in vitro measurements. ASAIO J. 54(1):64–72, 2008.
Park, Y., et al. Measurement of red blood cell mechanics during morphological changes. Proc. Natl. Acad. Sci. USA 107(15):6731–6736, 2010.
Pasqua, A., et al. Large-scale simulations of fluctuating biological membranes. J. Chem. Phys. 132(15):154107, 2010.
Pohl, H. A. The motion and precipitation of suspensoids in divergent electric fields. J. Appl. Phys. 22(7):869–871, 1951.
Pohl, H.A., Dielectrophoresis: applications to the characterization and separation of cells. In: Methods of Cell Separation, N. Catsimpoolas, ed., New York: CRC Press, 1977.
Pohl, H. A. Dielectrophoresis: The Behavior of Neutral Matter in Nonuniform Electric Fields, Vol. 80. Cambridge: University Press Cambridge, 1978.
Pohl, H. A., and J. S. Crane. Dielectrophoresis of cells. Biophys. J. 11(9):711, 1971.
Pommer, M. S., et al. Dielectrophoretic separation of platelets from diluted whole blood in microfluidic channels. Electrophoresis 29(6):1213–1218, 2008.
Radmacher, M., et al. Measuring the viscoelastic properties of human platelets with the atomic force microscope. Biophys. J. 70(1):556–567, 1996.
Ramos, A., et al. AC electrokinetics: a review of forces in microelectrode structures. J. Phys. D-Appl. Phys. 31(18):2338–2353, 1998.
Rand, R. P. Mechanical properties of the red cell membrane. Ii. Viscoelastic breakdown of the membrane. Biophys. J. 4:303–316, 1964.
Robert, O., M. J. B. Ritchie, and Paul Hansma. Plasticity and toughness in bone. Phys. Today 62(2):41–47, 2009.
Santos, S. F. D., and J. D. A. Rodrigues. Correlation between fracture toughness, work of fracture and fractal dimensions of Alumina-mullite-zirconia composites. Mater. Res. 6(2):219–226, 2003.
Sato, M., M. J. Levesque, and R. M. Nerem. Micropipette aspiration of cultured bovine aortic endothelial-cells exposed to shear-stress. Arteriosclerosis 7(3):276–286, 1987.
Sheriff, J., et al. High-shear stress sensitizes platelets to subsequent low-shear conditions. Ann. Biomed. Eng. 38(4):1442–1450, 2010.
Sheriff, J., et al. Evaluation of shear-induced platelet activation models under constant and dynamic shear stress loading conditions relevant to devices (vol 41, pg 1279, 2013). Ann. Biomed. Eng. 41(12):2712, 2013.
Sims, P. J., et al. Complement proteins C5b-9 cause release of membrane vesicles from the platelet surface that are enriched in the membrane receptor for coagulation factor Va and express prothrombinase activity. J. Biol. Chem. 263(34):18205–18212, 1988.
Sun, M., et al. The effect of cellular cholesterol on membrane-cytoskeleton adhesion. J. Cell Sci. 120(Pt 13):2223–2231, 2007.
Tran, P. L., Valerio, L., Yamaguchi, J., Brengle, W., DeCook, T.E., Hutchinson, M., Sen, N., Bluestein, D., Slepian, M.J. Dimethyl Sulfoxide: A New Nemesis of Shear-Induced Platelet Activation. In: Nanoengineering for Medicine and Biology. 2014. San Francisco, CA.
Valerio, L. Multi-Perspective Investigation of the Effectiveness of Anti-Thrombotic Treatments in Association with Shear-Mediated Platelet Activation, 2014, Politechnico di Milano, Milan, Italy (unpublished Doctoral Dissertation).
Valero, A., T. Braschler, and P. Renaud. A unified approach to dielectric single cell analysis: impedance and dielectrophoretic force spectroscopy. Lab Chip 10(17):2216–2225, 2010.
Van Vliet, K. J., G. Bao, and S. Suresh. The biomechanics toolbox: experimental approaches for living cells and biomolecules. Acta Mater. 51(19):5881–5905, 2003.
Vieira-de-Abreu, A., et al. Platelets: versatile effector cells in hemostasis, inflammation, and the immune continuum. Semin. Immunopathol. 34(1):5–30, 2012.
Wang, X. J., X. B. Wang, and P. R. C. Gascoyne. General expressions for dielectrophoretic force and electrorotational torque derived using the Maxwell stress tensor method. J. Electrostat. 39(4):277–295, 1997.
Wang, X. B., et al. Dielectrophoretic manipulation of particles. IEEE Trans. Ind. Appl. 33(3):660–669, 1997.
Ward, M. D., and D. A. Hammer. A theoretical analysis for the effect of focal contact formation on cell-substrate attachment strength. Biophys. J. 64(3):936–959, 1993.
Wong, P. K., W. Tan, and C. M. Ho. Cell relaxation after electrodeformation: effect of latrunculin A on cytoskeletal actin. J. Biomech. 38(3):529–535, 2005.
Xu, W. W., et al. Cell stiffness is a biomarker of the metastatic potential of ovarian cancer cells. Plos One 7(10):e46609, 2012.
Yamaguchi, J., et al. Desensitization of DMSO-treated platelets to common agonists via membrane modulation. Faseb J. 28(1):598, 2014.
Zhang, J., et al. Nanosecond pulse electric field (nanopulse): a novel non-ligand agonist for platelet activation. Arch. Biochem. Biophys. 471(2):240–248, 2008.
Zhang, C., et al. Dielectrophoresis for manipulation of micro/nano particles in microfluidic systems. Anal. Bioanal. Chem. 396(1):401–420, 2010.
Zhang, P., et al. Multiscale particle-based modeling of flowing platelets in blood plasma using dissipative particle dynamics and coarse grained molecular dynamics. Cell. Mol. Bioeng. 7(4):552–574, 2014.
Funding
Funding was provided by NIH/NIBIB Quantum 1U01 EBO 12487.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Aleksander S. Popel oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Leung, S.L., Lu, Y., Bluestein, D. et al. Dielectrophoresis-Mediated Electrodeformation as a Means of Determining Individual Platelet Stiffness. Ann Biomed Eng 44, 903–913 (2016). https://doi.org/10.1007/s10439-015-1383-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-015-1383-7