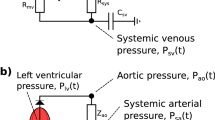
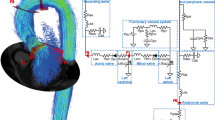
Abstract
A modeling approach integrated with a novel subject-specific characterization is here proposed for the assessment of hemodynamic values of the arterial tree. A 1D model is adopted to characterize large-to-medium arteries, while the left ventricle, aortic valve and distal micro-circulation sectors are described by lumped submodels. A new velocity profile and a new formulation of the non-linear viscoelastic constitutive relation suitable for the {Q, A} modeling are also proposed. The model is firstly verified semi-quantitatively against literature data. A simple but effective procedure for obtaining subject-specific model characterization from non-invasive measurements is then designed. A detailed subject-specific validation against in vivo measurements from a population of six healthy young men is also performed. Several key quantities of heart dynamics—mean ejected flow, ejection fraction, and left-ventricular end-diastolic, end-systolic and stroke volumes—and the pressure waveforms (at the central, radial, brachial, femoral, and posterior tibial sites) are compared with measured data. Mean errors around 5 and 8%, obtained for the heart and arterial quantities, respectively, testify the effectiveness of the model and its subject-specific characterization.




Similar content being viewed by others
References
Alastruey, J., A. W. Khir, K. S. Matthys, P. Segers, S. J. Sherwin, P. R. Verdonck, K. Parker, and J. Peirò. Pulse wave propagation in a model human arterial network: Assessment of 1-D visco-elastic simulations against in vitro measurements. J. Biomech. 44(12):2250–2258, 2011.
Avolio, A. P., Multi-branched model of the human arterial system. Med. Biol. Eng. Comput. 18(6):709–718, 1980.
Bessems, D., C. G. Giannopapa, M. C. M. Rutten, and F. N. van de Vosse. Experimental validation of a time-domain-based wave propagation model of blood flow in viscoelastic vessels. J. Biomech. 41(2):284–291, 2008.
Bessems, D., M. Rutten, and F. N. van de Vosse. A wave propagation model of blood flow in large vessels using an approximate velocity profile function. J. Fluid. Mech. 580:145–168, 2007.
Blanco, P. J., R. A. Feijóo, and S. Urquiza. A unified variational approach for coupling 3D–1D models and its blood flow applications. Comput. Methods Appl. Mech. 196(41–44):4391–4410, 2007.
Blanco, P.J., R.A. Feijóo. A dimensionally-heterogeneous closed-loop model for the cardiovascular system and its applications. Med. Eng. Phys. 35(5):652–667, 2013.
Blanco, P. J., P. R. Trenhago, L. G. Fernandes, and R. A. Feijóo. On the integration of the baroreflex control mechanism in a heterogeneous model of the cardiovascular system. Int. J. Numer. Methods Biomed. Eng., pp. 412–433, 2012.
Bollache, E., N. Kachenoura, A. Redheuil, F. Frouin, E. Mousseaux, P. Recho, and D. Lucor. Descending aorta subject-specific one-dimensional model validated against in vivo data. J. Biomech. 47(2):424–431, 2014.
Canic, S., C. Hartley, D. Rosenstrauch, J. Tambaca, G. Guidoboni, and A. Mikelić. Blood flow in compliant arteries: an effective viscoelastic reduced model, numerics, and experimental validation. Ann. Biomed. Eng. 34(4):575–592, 2006.
Caroli, A., S. Manini, L. Antiga, K. Passera, B. Ene-Iordache, R. Bogdan, S. Rota, G. Remuzzi, A. Bode, J. Leermakers, F.S. van de Vosse, R. Vanholder, M. Malovrh, J. Tordoir, and A. Remuzzi. Validation of a patient-specific hemodynamic computational model for surgical planning of vascular access in hemodialysis patients. Kidney Int. 84(6):1237–1245, 2013.
Chen, P., A. Quarteroni, and G. Rozza. Simulation-based uncertainty quantification of human arterial network hemodynamics. Int. J. Numer. Methods Biomed. Eng. 29(6):698–721, 2013.
Cockburn, B. An introduction to the discontinuous galerkin method for convection-dominated problems. In: Advanced Numerical Approximation of Nonlinear Hyperbolic Equations, edited by A. Quarteroni, Lecture Notes in Mathematics, vol. 1697, pp 151–268. Berlin: Springer, 1998.
Crouse, J. R., U. Goldbourt, G. Evans, J. Pinsky, A. R. Sharrett, P. Sorlie, W. Riley, G. Heiss, and for the ARIC Investigators. Risk factors and segment-specific carotid arterial enlargement in the atherosclerosis risk in communities (aric) cohort. Stroke 27(1):69–75, 1996.
Davis, A., C. Holloway, A. J. Lewandowski, N. Ntusi, R. M. Nethononda, A. Pitcher, J. M. Francis, P. Leeson, S. Neubauer, and O. J. Rider. Diameters of the normal thoracic aorta measured by cardiovascular magnetic resonance imaging; correlation with gender, body surface area and body mass index.J. Cardiovasc. Magn. Reson. 15(1):E77, 2013.
Fitchett, D. H. Lv-arterial coupling: interactive model to predict effect of wave reflections on lv energetics. Am. J. Physiol. Heart Circ. 261(4):1026–1033, 1991.
Formaggia, L., D. Lamponi, and A. Quarteroni. One dimensional models for blood flow in arteries. J. Eng. Math. 47(3–4):251–276, 2003.
Guyton, A., and J. Hall. Textbook of Medical Physiology, 11th edition. Philadelphia: Elsevier Saunders, 2004.
Hamburg, N. M., M. M. Mott, S. J. Bigornia, M.-A. Duess, M. A. Kluge, D. T. Hess, C. M. Apovian, J. A. Vita, and N. Gokce. Maladaptive enlargement of the brachial artery in severe obesity is reversed with weight loss. Vasc. Med. 15(3):215–222, 2010
Holenstein, A., P. Niederer, and M. Anliker. A viscoelastic model for use in predicting arterial pulse waves. J. Biomech. Eng. 102:318–326, 1980.
Huberts, W., C. de Jonge, W. van der Linden, M. Inda, J. Tordoir, F. van de Vosse, and E. Bosboom. A sensitivity analysis of a personalized pulse wave propagation model for arteriovenous fistula surgery. part a: Identification of most influential model parameters. Med. Eng. Phys. 35(6):810–826, 2013.
Kalita, P., and R. Schaefer. Mechanical Models of Artery Walls. Arch. Comput. Methods Eng. 15(1):1–36, 2007.
Kivity, Y., and R. Collins. Non linear wave propagation in viscoelastic tubes: application to aortic rupture. J. Biomech. 7:67–76, 1974.
Korakianitis, T., and Y. Shi. Numerical simulation of cardiovascular dynamics with healthy and diseased heart valves.J. Biomech. 39(11):1964–1982, 2006.
Krejza, J., M. Arkuszewski, S. E. Kasner, J. Weigele, A. Ustymowicz, R. W. Hurst, B. L. Cucchiara, and S. R. Messe. Carotid artery diameter in men and women and the relation to body and neck size. Stroke, 37(4):1103–1105, 2006.
Ku D. N. Blood flow in arteries. Annu. Rev. Fluid. Mech. 29:399–434, 1997.
Langewouters, G. Visco-elasticity of the human aorta in vitro in relation to pressure and age. PhD thesis, Free University, Amsterdam, 1982.
Liang, F., S. Takagi, R. Himeno, and H. Liu. Biomechanical characterization of ventricular-arterial coupling during aging: a multi-scale model study. J. Biomech. 42(6):692–704, 2009.
Lighthill, J. Mathematical Biofluiddynamics. Philadelphia, PA: Society for Industrial and Applied Mathematics, 1975.
Malossi, A. C. I., P. J. Blanco, and S. Deparis. A two-level time step technique for the partitioned solution of one-dimensional arterial networks. Comput. Methods Appl. Mech. 237–240:212–226, 2011.
Matthys, K.S., J. Alastruey, J. Peirò, A. W. Khir, P. Segers, P. R. Verdonck, K. Parker, and S. J. Sherwin. Pulse wave propagation in a model human arterial network: assessment of 1-d numerical simulations against in vitro measurements.J. Biomech. 40(15):3476–86, 2007.
Müller, L.O., and E. F. Toro. A global multiscale mathematical model for the human circulation with emphasis on the venous system. Int. J. Numer. Methods Biomed. Eng. 2014.
Mynard, J. P., M. R. Davidson, D. J. Penny, and J. J. Smolich. A simple, versatile valve model for use in lumped parameter and one-dimensional cardiovascular models. Int. J. Numer. Methods Biomed. Eng. 28: 626–641, 2012.
Nichols, W., and M. F. O’Rourke. McDonald’s Blood Flow in Arteries. Theoretical, Experimental and Clinical Principles. Oxford, UK: Oxford University Press, 2005.
Nicosia, S. and G. Pezzinga. Mathematical models of blood flow in the arterial network.J. Hydraul. Res. 45(2):188–201, 2007.
Noordergraaf, A., P. Verdouw, and H. Boom. The use of an analog computer in a circulation mode. Prog. Cardiovasc. Dis. 5:419–439, 1963.
Olufsen, M.S. Structured tree outflow condition for blood flow in larger systemic arteries. Am. J. Physiol. Heart Circ., 1999, pp. 257–268.
Olufsen, M. S., C. S. Peskin, W. Y. Kim, E. M. Pedersen, A. Nadim, and J. Larsen. Numerical simulation and experimental validation of blood flow in arteries with structured-tree outflow conditions. Ann. Biomed. Eng. 28(11):1281–99, 2000.
Ottesen, J., M. S. Olufsen, and J. K. Larsen. Applied Mathematical Models in Human Physiology, Vol. 33. Philadelphia, PA: Society for Industrial and Applied Mathematics, 2005.
Quarteroni, A. Cardiovascular Mathematics: Modeling and Simulation of the Circulatory System. MSA. Milano, Italy: Springer-Verlag, 2009.
Raghu, R., I. E. Vignon-Clementel, C. A. Figueroa, and C. A. Taylor. Comparative study of viscoelastic arterial wall models in nonlinear one-dimensional finite element simulations of blood flow. J. Biomech. Eng. 133(August):1–11, 2011.
Reymond, P., Y. Bohraus, F. Perren, F. Lazeyras, and N. Stergiopulos. Validation of a patient-specific one-dimensional model of the systemic arterial tree. Am. J. Physiol. Heart Circ. 301(3):1173–1182, 2011.
Reymond, P., F. Merenda, F. Perren, D. Rüfenacht, and N. Stergiopulos. Validation of a one-dimensional model of the systemic arterial tree. Am. J. Physiol. Heart Circ. 297(1):208–222, 2009.
Ruan, L., W. Chen, S. R. Srinivasan, M. Sun, H. Wang, A. Toprak, and G. S. Berenson. Correlates of common carotid artery lumen diameter in black and white younger adults: the bogalusa heart study. Stroke 40(3):702–707, 2009.
Sagawa, K. Cardiac Contraction and the Pressure–Volume Relationship. Oxford, UK: Oxford University Press, 1988.
Segers, P., N. Stergiopulos, N. Westerhof, P. Kolh, and P. R. Verdonck. Systemic and pulmonary hemodynamics assessed with a lumped-parameter heart-arterial interaction model. J. Eng. Math. 47:185–199, 2003.
Shi, Y., P. Lawford, and R. Hose. Review of zero-D and 1-D models of blood flow in the cardiovascular system. Biomed. Eng. Online 10(1):33, 2011.
Shishido, T., K. Hayashi, K. Shigemi, T. Sato, M. Sugimachi, and K. Sunagawa. Single-beat estimation of end-systolic elastance using bilinearly approximated time-varying elastance curve. Circulation 102(16):1983–1989, 2000.
Smulyan, H., S. J. Marchais, B. Pannier, A. P. Guerin, M. E. Safar, and G. M. London. Influence of body height on pulsatile arterial hemodynamic data.J. Am. Coll. Cardiol. 31(5):1103–1109, 1998.
Stergiopulos, N., D. F. Young, and T. R. Rogge. Computer simulation of arterial flow with applications to arterial and aortic stenoses. J. Biomech. 25(12):1477–1488, 1992.
Valdez-Jasso, D., D. Bia, Y. Zocalo, R. Armentano, M. A. Haider, and M. S. Olufsen. Linear and nonlinear viscoelastic modeling of aorta and carotid pressure-area dynamics under in vivo and ex vivo conditions. Ann. Biomed. Eng. 39(5):1438–1456, 2011.
van de Vosse, F.N., and N. Stergiopulos. Pulse wave propagation in the arterial tree. Annu. Rev. Fluid. Mech. 43(1):467–499, 2011.
Westerhof, N., F. Bosman, C. De Vriens, and A. Noordergraaf. Analog studies of the human systemic arterial tree. J. Biomech. 2(2):121–134, 1969.
Westerhof, N., N. Stergiopulos, and M. I. Noble. Snapshots of Hemodynamics: An Aid for Clinical Research and Graduate Education. New York: Springer, 2010.
Wildman, R. P., V. Mehta, T. Thompson, S. Brockwell, and K. Sutton-Tyrrell. Obesity is associated with larger arterial diameters in caucasian and African-American young adults. Diabetes Care 27(12):2997–2999, 2004.
Xiao, N., J. Alastruey, and C. A. Figueroa. Systematic comparison between 1-D and 3-D hemodynamics in compliant arterial models. Int. J. Numer. Methods Biomed. Eng. 30:204–231, 2014.
Zebekakis, P. E., T. Nawrot, L. Thijs, E. J. Balkestein, J. van der Heijden-Spek, L. M. Van Bortel, H. A. Struijker-Boudier, M. E. Safar, and J. A. Staessen. Obesity is associated with increased arterial stiffness from adolescence until old age. J. Hypertens. 23(10):1839–1846, 2005.
Acknowledgments
Franco Veglio, Alberto Milan, and Dario Leone of the of the Hypertension Center, Department of Medical Sciences, University of Turin are acknowledged for the data measure and their irreplaceable help.
Conflict of interest
There are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Diego Gallo oversaw the review of this article.
Appendices
Appendix
Coefficients of the constitutive Eq. (13)
Numerical Method
The model described in the second section is solved using the Runge-Kutta Discontinuous-Galerkin method12: first, space is discretized by a Discontinuous-Galerkin approach, then time evolution is described by a second-order Runge-Kutta scheme.
Conservative Form
In the conservative form, model (1) and (17) reads
where \(\mathbf {U}=[A(x,t),Q(x,t)]^T\) are the conservative variables, whereas the corresponding fluxes and source terms are
where \(b_x=g \sin (\gamma )\) is the longitudinal projection of the gravitational acceleration.
To solve system (29) in a one-dimensional generic domain \(\Omega \) discretized into \(N_{el}\) elemental non-overlapping regions \(\Omega _e=[x_e^l,x_e^r]\)—such that \(x_{e+1}^l=x_e^r\) for \(e=1,\ldots,N_{el}\) and \( \cup _{e=1}^{N_{el}} \Omega _e=\Omega \)—the weak form of Eq. (29) is written
where \(\varvec{\psi }\) is a set of arbitrary test functions in \(\Omega \) and \( (\mathbf {v},\mathbf {p})_\Omega =\int _\Omega \mathbf {v}\mathbf {p} \text {dx} \) is the standard \(\mathbf {L}^2(\Omega )\) inner product between two generic function \(\mathbf {v}\) and \(\mathbf {p}\) whose domain is the closed interval \(\Omega \). Decomposing the integrals in Eq. (30) into the elemental regions and integrating by part the second term, one obtains
In order to discretize the problem in space, U is assumed to belong to the finite dimensional space of the \(\mathbf {L}^2(\Omega )\) functions—that are polynomials of degree 1 on each element \(\Omega _e\)—obtaining the approximate solution \(\mathbf {U}_h\) (subscript \(h\) marks an element of such a space). Even if the solution \(\mathbf {U}_h\) can be discontinuous at boundaries between elemental regions, information propagates by upwinding the fluxes \(\mathbf {F}\) on the third term of Eq. (31).
The approximate solution \(\mathbf {U}_h=\mathbf {U}_h(x,t)\) on the element \(\Omega _e\) can be represented as
where \(\alpha _i\) are the time dependent unknown weights, \(\phi _i(\xi )\) are the trial functions on the reference element \(\hat{\Omega }=[-1,1]\), and the following affine mapping between \(\hat{\Omega }\) and \(\Omega _e\) is introduced
We choose first-degree Lagrange functions as expansion polynomial basis, \(\phi _1(\xi )\!=\!(1-\xi )/2\), \(\phi _2(\xi )\!=\!(1+\xi )/2\), and, following the usual Galerkin approach, the discrete test functions \(\varvec{\psi }_h\) are chosen in the same discrete space as the numerical solution \(\mathbf {U}_h\).
Introducing the approximate solution \(\mathbf {U}_h\) and the discrete test function into relation (31), one obtains the weak formulation as
where \(\mathbf {F}_{LF}\) are the upwinded numerical fluxes. They are obtained using the Lax-Friedrichs method as
where \(\mathbf {U}^+\) (\(\mathbf {U}^-\), resp.) are the variables on the right (left, resp.) side of the boundary and \(\lambda _{max}\) is the maximum eigenvalue of the matrix \(\mathbf {H}(\mathbf {U})\) of the quasi-linear form (see eq. (7.2.1), below).
The formulation (32) is then advanced in time by a second-order Runge-Kutta explicit method. The choice of such a low-order time-advancing scheme is coherent with the spatial discretization, in which the order of the expansion polynomial is one. This method is stable if the well-known Courant-Friedrichs-Lewy (CFL) condition is satisfied.
Boundary Conditions
When characteristics have slopes of opposite signs—as in the present situation, see below –, the differential system (1) and (17) needs only one physical condition at each domain boundary. However, the solution of the numerical problem requires to prescribe both variables at each boundary. For this reason, extra relations (called compatibility equations) have to be written at each boundary by projecting the differential equations along the outgoing characteristics.
Quasi-Linear Form
In order to formulate the compatibility conditions, system (29) is written in the quasi-linear form
where the terms
are obtained by neglecting viscoelasticity. Notice that the characteristic variables move along the corresponding characteristic directions for a spatial distance equal to the product of the time step by the local wave celerity. Being both time step and celerity small, the spatial distance is very small thus allowing the viscoelastic effect to be neglected.
Let \(\varvec{\Lambda }\) and \(\mathbf {L}\) be the eigenvalue and left eigenvector matrices of \(\mathbf {H}(\mathbf {U})\), respectively, so that \(\mathbf {L} \mathbf {H} \mathbf {L}^{-1} = \varvec{\Lambda }\). They read
In the present case, the eigenvalues read
where
is the celerity of the propagation when the fluid is at rest. At least in physiological condition, the system results strictly hyperbolic because \(c\) is always greater than \(\beta Q/A\), entailing \(\lambda _1<0\) (backward propagation) and \(\lambda _2>0\) (forward propagation).
Compatibility Conditions
The characteristic variables \(\mathbf {W}\) are defined as
Due to the structure of the matrix \(\mathbf {L}\), it is not possible to obtain the characteristic variables analytically. Therefore, pseudo-characteristic variables are introduced by linearising Eq. (35).
By pre-multiplying the vectorial Eq. (33) by the left eigenvector matrix, one obtains
and then
In order to introduce the total derivative, the first term of Eq. (36) is rewritten as
and the second term as
obtaining
By introducing the total derivative \(\mathrm{D} / \mathrm{D} t\), the last equation becomes
If time is now discretized so that \(\Delta t=t_{n+1}-t_{n}\) is the time step (the subscript \(n\) denotes the \(n\)-th time instant), the total derivative can be approximated as
where the superscript \(\star \) refers to the point located at the distance from the boundary equal to \(c \cdot \Delta t\). By assuming that
the departure of the pseudo-characteristic from a generic state \(\mathbf {U}_n^{\star }\) is taken as
where \(\mathbf {L}_n=\mathbf {L}(\mathbf {U}_n)\) and \(\varvec{\Lambda }_n=\varvec{\Lambda }(\mathbf {U}_n)\).
Finally, because the pseudo-characteristics at the initial state are set null, Eq. (39) needs to be balanced by subtracting the initial condition to the dependent variables,29 i.e.
where the subscript \(0\) refers to the initial condition. Since the reference condition (uniform pressure of 100 mmHg and no flow) is assumed as initial condition, the same subscript \(0\) is used to define both quantities.
The formulation (40) has to be coupled with the physical boundary conditions at every (internal and external) boundary. The corresponding non-linear systems are solved by an adaptive trust-region-dogleg method.
Rights and permissions
About this article
Cite this article
Guala, A., Camporeale, C., Tosello, F. et al. Modelling and Subject-Specific Validation of the Heart-Arterial Tree System. Ann Biomed Eng 43, 222–237 (2015). https://doi.org/10.1007/s10439-014-1163-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-014-1163-9