Abstract
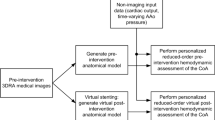
We propose a CFD-based approach for the non-invasive hemodynamic assessment of pre- and post-operative coarctation of aorta (CoA) patients. Under our approach, the pressure gradient across the coarctation is determined from computational modeling based on physiological principles, medical imaging data, and routine non-invasive clinical measurements. The main constituents of our approach are a reduced-order model for computing blood flow in patient-specific aortic geometries, a parameter estimation procedure for determining patient-specific boundary conditions and vessel wall parameters from non-invasive measurements, and a comprehensive pressure-drop formulation coupled with the overall reduced-order model. The proposed CFD-based algorithm is fully automatic, requiring no iterative tuning procedures for matching the computed results to observed patient data, and requires approximately 6–8 min of computation time on a standard personal computer (Intel Core2 Duo CPU, 3.06 GHz), thus making it feasible for use in a clinical setting. The initial validation studies for the pressure-drop computations have been performed on four patient datasets with native or recurrent coarctation, by comparing the results with the invasively measured peak pressure gradients recorded during routine cardiac catheterization procedure. The preliminary results are promising, with a mean absolute error of less than 2 mmHg in all the patients.





Similar content being viewed by others
Abbreviations
- c :
-
Wave speed
- C :
-
Windkessel compliance
- DBP/SBP:
-
Diastolic/systolic blood pressure
- E :
-
Young’s modulus
- HR :
-
Heart rate
- K v/K t/K u/K c :
-
Viscous/turbulent/inertance/continuous pressure-drop coefficient
- L c :
-
Coarctation length
- MAP:
-
Mean arterial pressure
- Q asc/Q desc :
-
Flow rate through the ascending/descending aorta
- Q CoA :
-
Flow rate through the coarctation
- Q supra-aortic :
-
Flow rate through supra-aortic vessels
- R c :
-
Coarctation resistance
- R d/R p/R t :
-
Distal/proximal/total Windkessel resistance
- Z:
-
Characteristic impedance
References
Arzani, A., P. Dyverfeldt, T. Ebbers, and S. Shadden. In vivo validation of numerical prediction for turbulence intensity in an aortic coarctation. Ann. Biomed. Eng. 40:860–870, 2012.
Bessems, D. On the Propagation of Pressure and Flow Waves Through the Patient-Specific Arterial System. PhD Thesis, TU Eindhoven, The Netherlands, 2007.
Coogan, J. S., F. P. Chan, C. A. Taylor, and J. A. Feinstein. Computational fluid dynamic simulations of aortic coarctation comparing the effects of surgical- and stent-based treatments on aortic compliance and ventricular workload. Catheter. Cardiov. Interv. 77:680–691, 2011.
Formaggia, L., D. Lamponi, M. Tuveri, and A. Veneziani. Numerical modeling of 1D arterial networks coupled with a lumped parameters description of the heart. Comput. Method. Biomech. 9:273–288, 2006.
Garcia, D., P. Pibarot, and L. G. Duranda. Analytical modeling of the instantaneous pressure gradient across the aortic valve. J. Biomech. 38:1303–1311, 2005.
Hom, J. J., K. Ordovas, and G. P. Reddy. Velocity-encoded cine MR imaging in aortic coarctation: functional assessment of hemodynamic events. Radiographics 28:407–416, 2008.
Ibrahim, E. S., K. Johnson, A. Miller, J. Shaffer, and R. White. Measuring aortic pulse wave velocity using high-field cardiovascular magnetic resonance: comparison of techniques. J. Cardiovasc. Magn. Reson. 12:26–38, 2010.
Kadem, L., D. Garcia, L. G. Durand, R. Rieu, J. G. Dumesnil, and P. Pibarot. Value and limitations of peak-to-peak gradient for evaluation of aortic stenosis. J. Heart Valve Dis. 15:609–616, 2006.
Keshavarz-Motamed, Z., J. Garcia, N. Maftoon, E. Bedard, P. Chetaille, and L. Kadem. A new approach for the evaluation of the severity of coarctation of the aorta using Doppler velocity index and effective orifice area: in vitro validation and clinical implications. J. Biomech. 45:1239–1245, 2012.
Keshavarz-Motamed, Z., J. Garcia, P. Pibarot, E. Larose, and L. Kadem. Modeling the impact of concomitant aortic stenosis and coarctation of the aorta on left ventricular workload. J. Biomech. 44:2817–2825, 2011.
LaDisa, J. F. J., C. A. Figueroa, I. E. Vignon-Clementel, H. J. Kim, N. Xiao, L. M. Ellwein, F. P. Chan, J. A. Feinstein, and C. A. Taylor. Computational simulations for aortic coarctation: representative results from a sampling of patients. J. Biomech. Eng. 133:091008, 2011.
Menon, A., D. C. Wendell, H. Wang, T. Eddinger, J. Toth, R. Dholakia, P. Larsen, E. Jensen, and J. F. J. LaDisa. A coupled experimental and computational approach to quantify deleterious, hemodynamics, vascular alterations, and mechanisms of long-term morbidity in response to aortic coarctation. J. Pharmacol. Toxicol. Methods 65:18–28, 2011.
Mynard, J. P., and P. Nithiarasu. A 1D arterial blood flow model incorporating ventricular pressure, aortic valve and regional coronary flow using the locally conservative Galerkin (LCG) method. Int. J. Numer. Method. Biomed. Eng. 24:367–417, 2008.
Olufsen, M., and C. Peskin. Numerical simulation and experimental validation of blood flow in arteries with structured-tree outflow conditions. Ann. Biomed. Eng. 28:1281–1299, 2000.
Ralovich, K., L. Itu, V. Mihalef, P. Sharma, R. Ionasec, D. Vitanovski, W. Krawtschuk, A. Everett, R. Ringel, N. Navab, and D. Comaniciu. Hemodynamic assessment of pre- and post-operative aortic coarctation from MRI. Proceedings of MICCAI, Nice, France, October 2012.
Razminia, M., A. Trivedi, J. Molnar, M. Elbzour, M. Guerrero, Y. Salem, A. Ahmed, S. Khosla, and D. L. Lubell. Validation of a new formula for mean arterial pressure calculation: the new formula is superior to the standard formula. Catheter. Cardiov. Interv. 63:419–425, 2004.
Reymond, P., Y. Bohraus, F. Perren, F. Lazeyras, and N. Stergiopulos. Validation of a patient-specific one-dimensional model of the systemic arterial tree. Am. J. Physiol. Heart C. 301:1173–1182, 2011.
Ringel, R. E., and K. Jenkins. Coarctation of the aorta stent trial (coast), 2007. http://clinicaltrials.gov/ct2/show/NCT00552812. Accessed March 10, 2012.
Seeley, B. D., and D. F. Young. Effect of geometry on pressure losses across models of arterial stenoses. J. Biomech. 9:439–448, 1976.
Seifert, B. L., K. DesRochers, M. Ta, G. Giraud, M. Zarandi, M. Gharib, and D. J. Sahn. Accuracy of Doppler methods for estimating peak-to-peak and peak instantaneous gradients across coarctation of the aorta: an In vitro study. J. Am. Soc. Echocardiogr. 12:744–753, 1999.
Steele, B. N., J. Wan, J. P. Ku, T. J. R. Hughes, and C. A. Taylor. In vivo validation of a one-dimensional finite-element method for predicting blood flow in cardiovascular bypass grafts. IEEE Trans. Biomed. Eng. 50:649–656, 2003.
Stergiopulos, N., D. F. Young, and T. R. Rogge. Computer simulation of arterial flow with applications to arterial and aortic Stenoses. J. Biomech. 25:1477–1488, 1992.
Valverde, I., C. Staicu, H. Grotenhuis, A. Marzo, K. Rhode, Y. Shi, A. Brown, A. Tzifa, T. Hussain, G. Greil, P. Lawford, R. Razavi, R. Hose, and P. Beerbaum. Predicting hemodynamics in native and residual coarctation: preliminary results of a rigid-wall computational-fluid-dynamics model validated against clinically invasive pressure measures at rest and during pharmacological stress. J. Cardiovasc. Magn. Reson. 13:49, 2011.
Vignon-Clementel, I., C. A. Figueroa, K. Jansen, and C. A. Taylor. Outflow boundary conditions for 3D simulations of non-periodic blood flow and pressure fields in deformable arteries. Comput. Methods Biomech. Biomed. Eng. 13:625–640, 2010.
Vitanovski, D., K. Ralovich, R. Ionasec, Y. Zheng, M. Suehling, W. Krawtschuk, J. Hornegger, and D. Comaniciu. Personalized learning-based segmentation of thoracic aorta and main branches for diagnosis and treatment planning. 9th IEEE International Symposium on Biomedical Imaging, Barcelona, Spain, 2012.
Willett, N., R. Long, K. Maiellaro-Rafferty, R. Sutliff, R. Shafer, J. Oshinski, D. Giddens, R. Guldberg, and R. Taylor. An in vivo murine model of low-magnitude oscillatory wall shear stress to address the molecular mechanisms of mechanotransduction. Arterioscler. Thromb. Vasc. Biol. 30:2099–2102, 2010.
Young, D., and F. Tsai. Flow characteristics in models of arterial stenoses—II. Unsteady flow. J. Biomech. 6:547–559, 1973.
Zamir, M., P. Sinclair, and T. H. Wonnacott. Relation between diameter and flow in major branches of the arch of the aorta. J. Biomech. 25:1303–1310, 1992.
Acknowledgments
The authors would like to acknowledge Dr. Michael Suehling and Dr. Constantin Suciu. This work was partially supported by the Sectorial Operational Programme Human Resources Development (SOP HRD), financed from the European Social Fund and by the Romanian Government under the contract number POSDRU/88/1.5/S/76945. This work has been partially funded by European Union project Sim-e-Child (FP7 – 248421).
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Scott L. Diamond oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Itu, L., Sharma, P., Ralovich, K. et al. Non-Invasive Hemodynamic Assessment of Aortic Coarctation: Validation with In Vivo Measurements. Ann Biomed Eng 41, 669–681 (2013). https://doi.org/10.1007/s10439-012-0715-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-012-0715-0