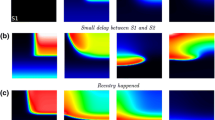
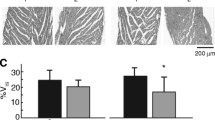
Abstract
Experimental results have shown that action potential (AP) conduction in ventricular tissue from streptozotocin-diabetic (STZ) rats is compromised. This was manifest as increased sensitivity of conduction velocity (CV) to the gap junction uncoupler heptanol, as well as increased sensitivity of CV to reduced cellular excitability due to elevated extracellular K+ concentration, in the STZ hearts. This “reduced conduction reserve” has been suggested to be due to lateralization of connexin43 (Cx43) proteins, rendering them nonfunctional, resulting in compromised intercellular electrical coupling. In this study, we have used computer simulations of one-dimensional AP conduction in a model of rat ventricular myocytes to verify this interpretation. Our results show that compromised intercellular coupling indeed reduces conduction reserve and predict a response to gap junction uncoupling with heptanol that is consistent with experiments. However, our simulations also show that compromised intercellular coupling is insufficient to explain the increased sensitivity to reduced cellular excitability. A thorough investigation of possible underlying mechanisms, suggests that subtle alterations in the voltage-dependence of steady-state gating for the Na+ current (I Na), combined with compromised intercellular coupling, is a likely mechanism for these observations.






Similar content being viewed by others
References
Abo, K., Y. Ishida, R. Yoshida, T. Hozumi, H. Ueno, H. Shiotani, K. Matsunaga, and T. Kazumi. Torsade de pointes in NIDDM with long QT intervals. Diabetes Care 19:1010, 1996.
Ashraf, A., and A. Nygren. Cardiac action potential wavefront tracking using optical mapping. In: Proceedings of the 31st Annual International Conference of the IEEE-EMBS, Minneapolis, MN, September 2–6, 2009, pp. 1766–1769.
Bhatnagar, A., S. K. Srivastava, and G. Szabo. Oxidative stress alters specific membrane currents in isolated cardiac myocytes. Circ. Res. 67:535–549, 1990.
Christensen, P. K., M. A. Gall, A. Major-Pedersen, A. Sato, P. Rossing, L. Breum, A. Pietersen, J. Kastrup, and H. H. Parving. QTc interval length and QT dispersion as predictors of mortality in patients with non-insulin-dependent diabetes. Scand. J. Clin. Lab. Invest. 60:323–332, 2000.
de Groot, J. R., and R. Coronel. Acute ischemia-induced gap junctional uncoupling and arrhythmogenesis. Cardiovasc. Res. 62:323–334, 2004.
De Mello, W. C. Impaired cell communication in the diabetic heart. The role of the renin angiotensin system. Mol. Cell Biochem. 296:53–58, 2007.
Desplantez, T., E. Dupont, N. J. Severs, and R. Weingart. Gap junction channels and cardiac impulse propagation. J. Membr. Biol. 218:13–28, 2007.
El-Atat, F. A., S. I. McFarlane, J. R. Sowers, and J. T. Bigger. Sudden cardiac death in patients with diabetes. Curr. Diab. Rep. 4:187–193, 2004.
Fang, Z. Y., J. B. Prins, and T. H. Marwick. Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr. Rev. 25:543–567, 2004.
Fonarow, G. C. An approach to heart failure and diabetes mellitus. Am. J. Cardiol. 96:47E–52E, 2005.
Ghaly, H., P. Boyle, E. Vigmond, and A. Nygren. Reduced conduction reserve of the propagating cardiac impulse in the diabetic rat heart: a model study. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2008:5926–5929, 2008.
Jongsma, H. J., and R. Wilders. Gap junctions in cardiovascular disease. Circ. Res. 86:1193–1197, 2000.
Kagiyama, Y., J. L. Hill, and L. S. Gettes. Interaction of acidosis and increased extracellular potassium on action potential characteristics and conduction in guinea pig ventricular muscle. Circ. Res. 51:614–623, 1982.
Kanno, S., and J. E. Saffitz. The role of myocardial gap junctions in electrical conduction and arrhythmogenesis. Cardiovasc. Pathol. 10:169–177, 2001.
Lerner, D. L., K. A. Yamada, R. B. Schuessler, and J. E. Saffitz. Accelerated onset and increased incidence of ventricular arrhythmias induced by ischemia in Cx43-deficient mice. Circulation 101:547–552, 2000.
Lin, X., M. Crye, and R. D. Veenstra. Regulation of connexin43 gap junctional conductance by ventricular action potentials. Circ. Res. 93:e63–e73, 2003.
Lin, H., K. Ogawa, I. Imanaga, and N. Tribulova. Remodeling of connexin 43 in the diabetic rat heart. Mol. Cell. Biochem. 290:69–78, 2006.
Luo, A., J. Ma, P. Zhang, H. Zhou, and W. Wang. Sodium channel gating modes during redox reaction. Cell Physiol. Biochem. 19:9–20, 2007.
McHowat, J., M. H. Creer, K. K. Hicks, J. H. Jones, R. McCrory, and R. H. Kennedy. Induction of Ca-independent PLA(2) and conservation of plasmalogen polyunsaturated fatty acids in diabetic heart. Am. J. Physiol. Endocrinol. Metab. 279:E25–E32, 2000.
Miyasaka, Y., M. E. Barnes, B. J. Gersh, S. S. Cha, K. R. Bailey, W. P. Abhayaratna, J. B. Seward, and T. S. Tsang. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 114:119–125, 2006.
Mooradian, A. D. Cardiovascular disease in type 2 diabetes mellitus: current management guidelines. Arch. Intern. Med. 163:33–40, 2003.
Morley, G. E., D. Vaidya, F. H. Samie, C. Lo, M. Delmar, and J. Jalife. Characterization of conduction in the ventricles of normal and heterozygous Cx43 knockout mice using optical mapping. J. Cardiovasc. Electrophysiol. 10:1361–1375, 1999.
Nygren, A., and W. R. Giles. Mathematical simulation of slowing of cardiac conduction velocity by elevated extracellular [K+] in a human atrial strand. Ann. Biomed. Eng. 28:951–957, 2000.
Nygren, A., and J. A. Halter. A general approach to modeling conduction and concentration dynamics in excitable cells of concentric cylindrical geometry. J. Theor. Biol. 199:329–358, 1999.
Nygren, A., M. L. Olson, K. Y. Chen, T. Emmett, G. Kargacin, and Y. Shimoni. Propagation of the cardiac impulse in the diabetic rat heart: reduced conduction reserve. J. Physiol. 580:543–560, 2007.
Pandit, S. V., R. B. Clark, W. R. Giles, and S. S. Demir. A mathematical model of action potential heterogeneity in adult rat left ventricular myocytes. Biophys. J. 81:3029–3051, 2001.
Pandit, S. V., W. R. Giles, and S. S. Demir. A mathematical model of the electrophysiological alterations in rat ventricular myocytes in type-I diabetes. Biophys. J. 84:832–841, 2003.
Rossing, P., L. Breum, A. Major-Pedersen, A. Sato, H. Winding, A. Pietersen, J. Kastrup, and H. H. Parving. Prolonged QTc interval predicts mortality in patients with Type 1 diabetes mellitus. Diabet. Med. 18:199–205, 2001.
Rudy, Y., and W. Quan. A model study of the effects of the discrete cellular structure on electrical propagation in cardiac tissue. Circ. Res. 61:815–823, 1987.
Rush, S., and H. Larsen. A practical algorithm for solving dynamic membrane equations. IEEE Trans. Biomed. Eng. 25:389–392, 1978.
Shander, G. S., A. I. Undrovinas, and J. C. Makielski. Rapid onset of lysophosphatidylcholine-induced modification of whole cell cardiac sodium current kinetics. J. Mol. Cell. Cardiol. 28:743–753, 1996.
Shang, L. L., S. Sanyal, A. E. Pfahnl, Z. Jiao, J. Allen, H. Liu, and S. C. Dudley, Jr. NF-kappaB-dependent transcriptional regulation of the cardiac scn5a sodium channel by angiotensin II. Am. J. Physiol. Cell Physiol. 294:C372–C379, 2008.
Shaw, R. M., and Y. Rudy. Electrophysiologic effects of acute myocardial ischemia: a mechanistic investigation of action potential conduction and conduction failure. Circ. Res. 80:124–138, 1997.
Shimoni, Y., T. Emmett, R. Schmidt, A. Nygren, and G. Kargacin. Sex-dependent impairment of cardiac action potential conduction in type 1 diabetic rats. Am. J. Physiol. Heart Circ. Physiol. 296:H1442–H1450, 2009.
Shimoni, Y., H. S. Ewart, and D. L. Severson. Type I and II models of diabetes produce different modifications of K+ currents in rat heart: role of insulin. J. Physiol. 507:485–496, 1998.
Shimoni, Y., L. Firek, D. L. Severson, and W. Giles. Short-term diabetes alters K+ currents in rat ventricular myocytes. Circ. Res. 74:620–628, 1994.
Shimoni, Y., D. Hunt, K. Chen, T. Emmett, and G. Kargacin. Differential autocrine modulation of atrial and ventricular potassium currents and of oxidative stress in diabetic rats. Am. J. Physiol. Heart Circ. Physiol. 290:H1879–H1888, 2006.
Shimoni, Y., D. Hunt, M. Chuang, K. Y. Chen, G. Kargacin, and D. L. Severson. Modulation of potassium currents by angiotensin and oxidative stress in cardiac cells from the diabetic rat. J. Physiol. 567:177–190, 2005.
Spach, M. S., J. F. Heidlage, R. C. Barr, and P. C. Dolber. Cell size and communication: role in structural and electrical development and remodeling of the heart. Heart Rhythm. 1:500–515, 2004.
Vigmond, E. J., and C. Clements. Construction of a computer model to investigate sawtooth effects in the Purkinje system. IEEE Trans. Biomed. Eng. 54:389–399, 2007.
Weingart, R., and F. F. Bukauskas. Long-chain n-alkanols and arachidonic acid interfere with the Vm-sensitive gating mechanism of gap junction channels. Pflugers Arch. 435:310–319, 1998.
Xu, Z., K. P. Patel, M. F. Lou, and G. J. Rozanski. Up-regulation of K(+) channels in diabetic rat ventricular myocytes by insulin and glutathione. Cardiovasc. Res. 53:80–88, 2002.
Zimmet, P., K. G. Alberti, and J. Shaw. Global and societal implications of the diabetes epidemic. Nature 414:782–787, 2001.
Acknowledgments
Operating funding from the Natural Sciences and Engineering Research Council (NSERC) and the Heart & Stroke Foundation of Canada is gratefully acknowledged. Patrick Boyle was supported by scholarships from NSERC and the Alberta Ingenuity Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghaly, H.A., Boyle, P.M., Vigmond, E.J. et al. Simulations of Reduced Conduction Reserve in the Diabetic Rat Heart: Response to Uncoupling and Reduced Excitability. Ann Biomed Eng 38, 1415–1425 (2010). https://doi.org/10.1007/s10439-009-9855-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-009-9855-2