Abstract
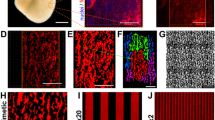
A novel cell culture methodology is described in which diffusion tensor magnetic resonance imaging (DTMRI) and cell micropatterning are combined to fabricate cell monolayers that replicate realistic cross-sectional tissue structure. As a proof-of-principle, neonatal rat ventricular myocyte (NRVM) monolayers were cultured to replicate the tissue microstructure of murine ventricular cross-sections. Specifically, DTMRI-measured in-plane cardiac fiber directions were converted into soft-lithography photomasks. Silicone stamps fabricated from the photomasks deposit fibronectin patterns to guide local cellular alignment. Fibronectin patterns consisted of a matrix of 190 μm2 subregions, each comprised of parallel lines 11–20 μm-wide, spaced 2–8.5 μm apart, and angled to match local DTMRI-measured fiber directions. Within 6 days of culture, NRVMs established confluent, electrically coupled monolayers, and for 18 μm-wide, 5 μm-spaced lines, directions of cell alignment in subregions microscopically replicated DTMRI-measurements with a local error of 7.2 ± 4.1°. By adjusting fibronectin line widths and spacings, cell elongation, gap junctional membrane distribution, and local cellular disarray were altered without changing the dominant directions of cell alignment in individual subregions. Changes in the anisotropy of electrical propagation were assessed by optically mapping membrane potentials. This novel methodology is expected to enable systematic studies of intramural structure–function relationships in both healthy and structurally remodeled hearts.






Similar content being viewed by others
References
Badie, N., and N. Bursac. Micropatterned heart slice cultures for studies of intramural cardiac electrophysiology. Paper presented at American Heart Association: Scientific Sessions, Chicago, IL, Nov 15, 2006.
Badie, N., and N. Bursac. Micropatterned ventricular slice: role of realistic tissue microstructure in impulse conduction. Paper presented at American Heart Association: Scientific Sessions, New Orleans, LA, 2008.
Badie, N., and N. Bursac. Novel micropatterned cardiac cell cultures with realistic ventricular microstructure. Biophys. J. 96(9):3873–3885, 2009.
Bub, G., K. Tateno, A. Shrier, and L. Glass. Spontaneous initiation and termination of complex rhythms in cardiac cell culture. J. Cardiovasc. Electrophysiol. 14(10 Suppl):S229–S236, 2003.
Bursac, N., F. Aguel, and L. Tung. Multiarm spirals in a two-dimensional cardiac substrate. Proc. Natl. Acad. Sci. USA 101(43):15530–15534, 2004.
Bursac, N., K. K. Parker, S. Iravanian, and L. Tung. Cardiomyocyte cultures with controlled macroscopic anisotropy. A model for functional electrophysiological studies of cardiac muscle. Circ. Res. 91:e45–e54, 2002.
Bursac, N., and L. Tung. Acceleration of functional reentry by rapid pacing in anisotropic cardiac monolayers: formation of multi-wave functional reentries. Cardiovasc. Res. 69(2):381–390, 2006.
Chen, J., S. K. Song, W. Liu, M. McLean, J. S. Allen, J. Tan, S. A. Wickline, and X. Yu. Remodeling of cardiac fiber structure after infarction in rats quantified with diffusion tensor MRI. Am. J. Physiol. Heart Circ. Physiol. 285(3):H946–H954, 2003.
de Diego, C., R. K. Pai, A. S. Dave, A. Lynch, M. Thu, F. Chen, L. H. Xie, J. N. Weiss, and M. Valderrabano. Spatially discordant alternans in cardiomyocyte monolayers. Am. J. Physiol. Heart Circ. Physiol. 294(3):H1417–H1425, 2008.
Entcheva, E., S. N. Lu, R. H. Troppman, V. Sharma, and L. Tung. Contact fluorescence imaging of reentry in monolayers of cultured neonatal rat ventricular myocytes. J. Cardiovasc. Electrophysiol. 11(6):665–676, 2000.
Fast, V. G., and R. E. Ideker. Simultaneous optical mapping of transmembrane potential and intracellular calcium in myocyte cultures. J. Cardiovasc. Electrophysiol. 11(5):547–556, 2000.
Fast, V. G., and A. G. Kleber. Cardiac tissue geometry as a determinant of unidirectional conduction block: assessment of microscopic excitation spread by optical mapping in patterned cell cultures and in a computer model. Cardiovasc. Res. 29(5):697–707, 1995.
Gao, X., X. He, B. Luo, L. Peng, J. Lin, and Z. Zuo. Angiotensin II increases collagen I expression via transforming growth factor-beta1 and extracellular signal-regulated kinase in cardiac fibroblasts. Eur. J. Pharmacol. 606(1–3):115–120, 2009.
Geerts, L., P. Bovendeerd, K. Nicolay, and T. Arts. Characterization of the normal cardiac myofiber field in goat measured with MR-diffusion tensor imaging. Am. J. Physiol. Heart Circ. Physiol. 283(1):H139–H145, 2002.
Grinnell, F., H. Fukamizu, P. Pawelek, and S. Nakagawa. Collagen processing, crosslinking, and fibril bundle assembly in matrix produced by fibroblasts in long-term cultures supplemented with ascorbic acid. Exp. Cell Res. 181(2):483–491, 1989.
Helm, P., M. F. Beg, M. I. Miller, and R. L. Winslow. Measuring and mapping cardiac fiber and laminar architecture using diffusion tensor MR imaging. Ann. NY Acad. Sci. 1047:296–307, 2005.
Holmes, A. A., D. F. Scollan, and R. L. Winslow. Direct histological validation of diffusion tensor MRI in formaldehyde-fixed myocardium. Magn. Reson. Med. 44(1):157–161, 2000.
Hooks, D. A., M. L. Trew, B. J. Caldwell, G. B. Sands, I. J. Legrice, and B. H. Smaill. Laminar arrangement of ventricular myocytes influences electrical behavior of the heart. Circ. Res., 2007.
Hsu, E. W., A. L. Muzikant, S. A. Matulevicius, R. C. Penland, and C. S. Henriquez. Magnetic resonance myocardial fiber-orientation mapping with direct histological correlation. Am. J. Physiol. 274(5 Pt 2):H1627–H1634, 1998.
Iravanian, S., Y. Nabutovsky, C. R. Kong, S. Saha, N. Bursac, and L. Tung. Functional reentry in cultured monolayers of neonatal rat cardiac cells. Am. J. Physiol. Heart Circ. Physiol. 285(1):H449–H456, 2003.
Jiang, Y., K. Pandya, O. Smithies, and E. W. Hsu. Three-dimensional diffusion tensor microscopy of fixed mouse hearts. Magn. Reson. Med. 52(3):453–460, 2004.
Karlon, W. J., J. W. Covell, A. D. McCulloch, J. J. Hunter, and J. H. Omens. Automated measurement of myofiber disarray in transgenic mice with ventricular expression of ras. Anat. Rec. 252(4):612–625, 1998.
Kawara, T., R. Derksen, J. de Groot, R. Coronel, S. Tasseron, A. Linnenbank, R. Hauer, H. Kirkels, M. Janse, and J. de Bakker. Activation delay after premature stimulation in chronically diseased human myocardium relates to the architecture of interstitial fibrosis. Circulation 104:3069–3075, 2001.
Klinger, R., and N. Bursac. Cardiac cell therapy in vitro: reproducible assays for comparing the efficacy of different donor cells. IEEE Eng. Med. Biol. Mag. 27(1):72–80, 2008.
Koura, T., M. Hara, S. Takeuchi, K. Ota, Y. Okada, S. Miyoshi, A. Watanabe, K. Shiraiwa, H. Mitamura, I. Kodama, and S. Ogawa. Anisotropic conduction properties in canine atria analyzed by high-resolution optical mapping: preferential direction of conduction block changes from longitudinal to transverse with increasing age. Circulation 105(17):2092–2098, 2002.
Kucera, J. P., A. G. Kleber, and S. Rohr. Slow conduction in cardiac tissue, II: effects of branching tissue geometry. Circ. Res. 83(8):795–805, 1998.
LeGrice, I. J., B. H. Smaill, L. Z. Chai, S. G. Edgar, J. B. Gavin, and P. J. Hunter. Laminar structure of the heart: ventricular myocyte arrangement and connective tissue architecture in the dog. Am. J. Physiol. 269(2 Pt 2):H571–H582, 1995.
Lijnen, P. J., V. V. Petrov, and R. H. Fagard. Induction of cardiac fibrosis by transforming growth factor-beta(1). Mol. Genet. Metab. 71(1–2):418–435, 2000.
Lim, Z. Y., B. Maskara, F. Aguel, R. Emokpae, Jr., and L. Tung. Spiral wave attachment to millimeter-sized obstacles. Circulation 114(20):2113–2121, 2006.
McSpadden, L. C., R. D. Kirkton, and N. Bursac. Electrotonic loading of anisotropic cardiac monolayers by unexcitable cells depends on connexin type and expression level. Am. J. Physiol. 2009.
Munoz, V., K. R. Grzeda, T. Desplantez, S. V. Pandit, S. Mironov, S. M. Taffet, S. Rohr, A. G. Kleber, and J. Jalife. Adenoviral expression of IKs contributes to wavebreak and fibrillatory conduction in neonatal rat ventricular cardiomyocyte monolayers. Circ. Res. 101(5):475–483, 2007.
Pedrotty, D. M., R. Y. Klinger, N. Badie, S. Hinds, A. Kardashian, and N. Bursac. Structural coupling of cardiomyocytes and noncardiomyocytes: quantitative comparisons using a novel micropatterned cell pair assay. Am. J. Physiol. Heart Circ. Physiol. 295(1):H390–H400, 2008.
Rohmer, D., A. Sitek, and G. T. Gullberg. Reconstruction and visualization of fiber and laminar structure in the normal human heart from ex vivo diffusion tensor magnetic resonance imaging (DTMRI) data. Invest. Radiol. 42(11):777–789, 2007.
Rohr, S., J. P. Kucera, and A. G. Kleber. Slow conduction in cardiac tissue, I: effects of a reduction of excitability versus a reduction of electrical coupling on microconduction. Circ. Res. 83(8):781–794, 1998.
Rohr, S., and B. Salzberg. Characterization of impulse propagation at the microscopic level across geometrically defined expansions of excitable tissue: multiple site optical recording of transmembrane voltage (MSORTV) in patterned growth heart cell cultures. J. Gen. Physiol. 104:287–309, 1994.
Schmid, P., T. Jaermann, P. Boesiger, P. F. Niederer, P. P. Lunkenheimer, C. W. Cryer, and R. H. Anderson. Ventricular myocardial architecture as visualised in postmortem swine hearts using magnetic resonance diffusion tensor imaging. Eur. J. Cardiothorac. Surg. 27(3):468–472, 2005.
Scollan, D. F., A. Holmes, R. Winslow, and J. Forder. Histological validation of myocardial microstructure obtained from diffusion tensor magnetic resonance imaging. Am. J. Physiol. 275(6 Pt 2):H2308–H2318, 1998.
Scollan, D. F., A. Holmes, J. Zhang, and R. L. Winslow. Reconstruction of cardiac ventricular geometry and fiber orientation using magnetic resonance imaging. Ann. Biomed. Eng. 28(8):934–944, 2000.
Spach, M. S., and J. P. Boineau. Microfibrosis produces electrical load variations due to loss of side-to-side cell connections: a major mechanism of structural heart disease arrhythmias. Pacing Clin. Electrophysiol. 20(2 Pt 2):397–413, 1997.
Spach, M. S., and P. C. Dolber. Relating extracellular potentials and their derivatives to anisotropic propagation at a microscopic level in human cardiac muscle. Evidence for electrical uncoupling of side-to-side fiber connections with increasing age. Circ. Res. 58(3):356–371, 1986.
Valderrabano, M. Influence of anisotropic conduction properties in the propagation of the cardiac action potential. Prog. Biophys. Mol. Biol. 94(1–2):144–168, 2007.
Wu, M. T., W. Y. Tseng, M. Y. Su, C. P. Liu, K. R. Chiou, V. J. Wedeen, T. G. Reese, and C. F. Yang. Diffusion tensor magnetic resonance imaging mapping the fiber architecture remodeling in human myocardium after infarction: correlation with viability and wall motion. Circulation 114(10):1036–1045, 2006.
Acknowledgments
The authors would like to thank Dr. Yi Jiang and Dr. Edward Hsu for supplying the DTMRI data, as well as Weining Bian for her assistance with the optical profile acquisition of the template wafer. This work was supported by the American Heart Association Predoctoral Fellowship (No. 0715178U) to Nima Badie.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Badie, N., Satterwhite, L. & Bursac, N. A Method to Replicate the Microstructure of Heart Tissue In Vitro Using DTMRI-Based Cell Micropatterning. Ann Biomed Eng 37, 2510–2521 (2009). https://doi.org/10.1007/s10439-009-9815-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-009-9815-x