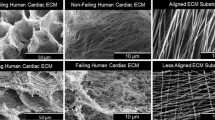
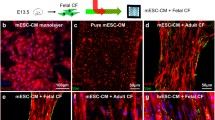
Abstract
Introduction
In native heart tissue, functions of cardiac fibroblasts (CFs) include synthesis, remodeling, and degradation of the extracellular matrix (ECM) as well as secreting factors that regulate cardiomyocyte (CM) function. The influence of direct co-culture and CF-derived ECM on CM mechanical function are not fully understood.
Methods
Here we use an engineered culture platform that provides control over ECM geometry and substrate stiffness to evaluate the influence of iPSC-CFs, and the ECM they produce, on the mechanical function of iPSC-CMs. Mechanical analysis was performed using digital image correlation to quantify maximum contractile strain, spontaneous contraction rate, and full-field organization of the contractions.
Results
When cultured alone, iPSC-CFs produce and remodel the ECM into fibers following the underlying 15° chevron patterned ECM. The substrates were decellularized and confirmed to have highly aligned fibers that covered a large fraction of the pattern area before reseeding with iPSC-CMs, alone or in co-culture with iPSC-CFs. When seeded on decellularized ECM, larger maximum contractile strains were observed in the co-culture condition compared to the CM Only condition. No significant difference was found in contractile strain between the Matrigel and decellularized ECM conditions; however, the spontaneous contraction rate was lower in the decellularized ECM condition. A methodology for quantifying alignment of cell contraction across the entire field of view was developed based on trajectories approximating the cell displacements during contraction. Trajectory alignment was unaltered by changes in culture or ECM conditions.
Conclusions
These combined observations highlight the important role CFs play in vivo and the need for models that enable a quantitative approach to examine interactions between the CFs and CMs, as well as the interactions of these cells with the ECM.







Similar content being viewed by others
References
Camelliti, P., T. K. Borg, and P. Kohl. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc. Res. 65(1):40–51, 2005. https://doi.org/10.1016/j.cardiores.2004.08.020.
MacKenna, D., S. R. Summerour, and F. J. Villarreal. Role of mechanical factors in modulating cardiac fibroblast function and extracellular matrix synthesis. Cardiovasc. Res. 46(2):257–263, 2000. https://doi.org/10.1016/S0008-6363(00)00030-4.
Fan, D., A. Takawale, J. Lee, and Z. Kassiri. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis Tissue Repair. 5(1):15, 2012. https://doi.org/10.1186/1755-1536-5-15.
Souders, C. A., S. L. K. Bowers, and T. A. Baudino. Cardiac fibroblast: the renaissance cell. Circ. Res. 105(12):1164–1176, 2009. https://doi.org/10.1161/CIRCRESAHA.109.209809.
Hall, C., K. Gehmlich, C. Denning, and D. Pavlovic. Complex relationship between cardiac fibroblasts and cardiomyocytes in health and disease. J Am. Heart Assoc.10(5):e019338, 2021. https://doi.org/10.1161/JAHA.120.019338.
Li, Y., H. Asfour, and N. Bursac. Age-dependent functional crosstalk between cardiac fibroblasts and cardiomyocytes in a 3D engineered cardiac tissue. Acta Biomater. 55:120–130, 2017. https://doi.org/10.1016/j.actbio.2017.04.027.
Porter, K. E., and N. A. Turner. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol. Ther. 123(2):255–278, 2009. https://doi.org/10.1016/j.pharmthera.2009.05.002.
Doppler, S. A., et al. Cardiac fibroblasts: more than mechanical support. J Thorac Dis. 9(Suppl 1):S36–S51, 2017. https://doi.org/10.21037/jtd.2017.03.122.
Zhang, D., I. Shadrin, J. Lam, H.-Q. Xian, R. Snodgrass, and N. Bursac. Tissue-engineered Cardiac Patch for Advanced Functional Maturation of Human ESC-derived Cardiomyocytes. Biomaterials. 34(23):5813–5820, 2013. https://doi.org/10.1016/j.biomaterials.2013.04.026.
Giacomelli, E., et al. Human-iPSC-derived cardiac stromal cells enhance maturation in 3D cardiac microtissues and reveal non-cardiomyocyte contributions to heart disease. Cell Stem Cell. 26(6):862-879.e11, Jun.2020. https://doi.org/10.1016/j.stem.2020.05.004.
Beauchamp, P., et al. 3D co-culture of hiPSC-derived cardiomyocytes with cardiac fibroblasts improves tissue-like features of cardiac spheroids. Front Mol Biosci. 7:14, Feb.2020. https://doi.org/10.3389/fmolb.2020.00014.
Napiwocki, B. N., et al. Micropattern platform promotes extracellular matrix remodeling by human PSC-derived cardiac fibroblasts and enhances contractility of co-cultured cardiomyocytes. Physiol. Rep.9(19):e15045, 2021. https://doi.org/10.14814/phy2.15045.
Pfannkuche, K., et al. Fibroblasts facilitate the engraftment of embryonic stem cell-derived cardiomyocytes on three-dimensional collagen matrices and aggregation in hanging drops. Stem Cells Dev. 19(10):1589–1599, Oct.2010. https://doi.org/10.1089/scd.2009.0255.
Kensah, G., et al. Murine and human pluripotent stem cell-derived cardiac bodies form contractile myocardial tissue in vitro. Eur. Heart J. 34(15):1134–1146, Apr.2013. https://doi.org/10.1093/eurheartj/ehs349.
Liau, B., N. Christoforou, K. Leong, and N. Bursac. Pluripotent stem cell-derived cardiac tissue patch with advanced structure and function. Biomaterials. 32(35):9180–9187, 2011. https://doi.org/10.1016/j.biomaterials.2011.08.050.
Thavandiran, N., et al. Design and formulation of functional pluripotent stem cell-derived cardiac microtissues. Proc. Natl. Acad. Sci. USA. 2013. https://doi.org/10.1073/pnas.1311120110.
Matsuura, K., et al. Creation of mouse embryonic stem cell-derived cardiac cell sheets. Biomaterials. 32(30):7355–7362, 2011. https://doi.org/10.1016/j.biomaterials.2011.05.042.
Kurose, H. Cardiac fibrosis and fibroblasts. Cells. 10(7):1716, 2021. https://doi.org/10.3390/cells10071716.
Kim, D.-H., et al. Nanoscale cues regulate the structure and function of macroscopic cardiac tissue constructs. Proc. Natl. Acad. Sci. USA. 107(2):565–570, 2010. https://doi.org/10.1073/pnas.0906504107.
Xing, Q., C. Vogt, K. W. Leong, and F. Zhao. Highly aligned nanofibrous scaffold derived from decellularized human fibroblasts. Adv. Funct. Mater. 24(20):3027–3035, 2014. https://doi.org/10.1002/adfm.201303460.
Ogle, B. M., et al. Distilling complexity to advance cardiac tissue engineering. Sci. Transl. Med. 8(342):342, 2016. https://doi.org/10.1126/scitranslmed.aad2304.
van Putten, S., Y. Shafieyan, and B. Hinz. Mechanical control of cardiac myofibroblasts. J. Mol. Cell. Cardiol. 93:133–142, 2016. https://doi.org/10.1016/j.yjmcc.2015.11.025.
Hazeltine, L. B., et al. Effects of substrate mechanics on contractility of cardiomyocytes generated from human pluripotent stem cells. Int J Cell Biol. 2012. https://doi.org/10.1155/2012/508294.
Ribeiro, A. J. S., et al. Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proc. Natl. Acad. Sci. USA. 112(41):12705–12710, 2015. https://doi.org/10.1073/pnas.1508073112.
Zhao, Y., et al. A platform for generation of chamber-specific cardiac tissues and disease modeling. Cell. Jan.2019. https://doi.org/10.1016/j.cell.2018.11.042.
Ronaldson-Bouchard, K., et al. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature. 2018. https://doi.org/10.1038/s41586-018-0016-3.
Shadrin, I. Y., et al. Cardiopatch platform enables maturation and scale-up of human pluripotent stem cell-derived engineered heart tissues. Nat. Commun. 8(1):1, 2017. https://doi.org/10.1038/s41467-017-01946-x.
Kim, D.-H., K. Han, K. Gupta, K. W. Kwon, K.-Y. Suh, and A. Levchenko. Mechanosensitivity of fibroblast cell shape and movement to anisotropic substratum topography gradients. Biomaterials. 30(29):5433–5444, 2009. https://doi.org/10.1016/j.biomaterials.2009.06.042.
Malte, Tiburcy, et al. Defined engineered human myocardium with advanced maturation for applications in heart failure modeling and repair. Circulation. 135(19):1832–1847, 2017. https://doi.org/10.1161/CIRCULATIONAHA.116.024145.
Nunes, S. S., et al. Biowire: a new platform for maturation of human pluripotent stem cell derived cardiomyocytes. Nat. Methods. 10(8):781–787, 2013. https://doi.org/10.1038/nmeth.2524.
Ruan, J.-L., et al. Mechanical stress conditioning and electrical stimulation promote contractility and force maturation of induced pluripotent stem cell-derived human cardiac tissue. Circulation. 134(20):1557–1567, 2016. https://doi.org/10.1161/CIRCULATIONAHA.114.014998.
Salick, M. R., et al. Micropattern width dependent sarcomere development in human ESC-derived cardiomyocytes. Biomaterials. 35(15):4454–4464, 2014. https://doi.org/10.1016/j.biomaterials.2014.02.001.
Napiwocki, B. N., et al. Aligned human cardiac syncytium for in vitro analysis of electrical, structural, and mechanical readouts. Biotechnol. Bioeng. 118(1):442–452, 2021. https://doi.org/10.1002/bit.27582.
Salick, M. R., B. N. Napiwocki, R. A. Kruepke, G. T. Knight, R. S. Ashton, and W. C. Crone. The scanning gradient Fourier transform (SGFT) method for assessing sarcomere organization and alignment. J. Appl. Phys.127(19):194701, 2020. https://doi.org/10.1063/1.5129347.
Lian, X., et al. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc. Natl. Acad. Sci. USA. 109(27):E1848–E1857, 2012. https://doi.org/10.1073/pnas.1200250109.
Tohyama, S., et al. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell. 12(1):127–137, Jan.2013. https://doi.org/10.1016/j.stem.2012.09.013.
Zhang, J., et al. Functional cardiac fibroblasts derived from human pluripotent stem cells via second heart field progenitors. Nat. Commun. 10(1):1–15, May2019. https://doi.org/10.1038/s41467-019-09831-5.
Palchesko, R. N., L. Zhang, Y. Sun, and A. W. Feinberg. Development of polydimethylsiloxane substrates with tunable elastic modulus to study cell mechanobiology in muscle and nerve. PLoS ONE.7(12):e51499, Dec.2012. https://doi.org/10.1371/journal.pone.0051499.
B. N. Napiwocki, M. R. Salick, R. S. Ashton, and W. C. Crone, “Controlling hESC-CM Cell Morphology on Patterned Substrates Over a Range of Stiffness,” in Mechanics of Biological Systems and Materials, Volume 6, C. S. Korach, S. A. Tekalur, and P. Zavattieri, Eds., in Conference Proceedings of the Society for Experimental Mechanics Series. Cham: Springer International Publishing, 2017, pp. 161–168. doi: https://doi.org/10.1007/978-3-319-41351-8_23.
Notbohm, J., et al. Two-dimensional culture systems to enable mechanics-based assays for stem cell-derived cardiomyocytes. Exp Mech. 59(9):1235–1248, 2019. https://doi.org/10.1007/s11340-019-00473-8.
Bar-Kochba, E., J. Toyjanova, E. Andrews, K.-S. Kim, and C. Franck. A fast iterative digital volume correlation algorithm for large deformations. Exp Mech. 55(1):261–274, 2015. https://doi.org/10.1007/s11340-014-9874-2.
Treloar, K. K., and M. J. Simpson. Sensitivity of edge detection methods for quantifying cell migration assays. PLoS ONE.8(6):e67389, 2013. https://doi.org/10.1371/journal.pone.0067389.
Naito, H., et al. Optimizing engineered heart tissue for therapeutic applications as surrogate heart muscle. Circulation. 114(1):1–72, 2006. https://doi.org/10.1161/CIRCULATIONAHA.105.001560.
Miragoli, M., G. Gaudesius, and S. Rohr. Electrotonic modulation of cardiac impulse conduction by myofibroblasts. Circ. Res. 98(6):801–810, 2006. https://doi.org/10.1161/01.RES.0000214537.44195.a3.
LaFramboise, W. A., et al. Cardiac fibroblasts influence cardiomyocyte phenotype in vitro. Am. J. Physiol. Cell Physiol. 292(5):C1799–C1808, 2007. https://doi.org/10.1152/ajpcell.00166.2006.
Liau, B., C. P. Jackman, Y. Li, and N. Bursac. Developmental stage-dependent effects of cardiac fibroblasts on function of stem cell-derived engineered cardiac tissues. Sci. Rep. 7(1):1, 2017. https://doi.org/10.1038/srep42290.
Sartiani, L., E. Bettiol, F. Stillitano, A. Mugelli, E. Cerbai, and M. E. Jaconi. Developmental changes in cardiomyocytes differentiated from human embryonic stem cells: a molecular and electrophysiological approach. Stem. Cells. 25(5):1136–1144, 2007. https://doi.org/10.1634/stemcells.2006-0466.
Snir, M., et al. Assessment of the ultrastructural and proliferative properties of human embryonic stem cell-derived cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 285(6):H2355–H2363, 2003. https://doi.org/10.1152/ajpheart.00020.2003.
Guo, Y., and W. T. Pu. Cardiomyocyte maturation. Circ. Res. 126(8):1086–1106, Apr.2020. https://doi.org/10.1161/CIRCRESAHA.119.315862.
Lundy, S. D., W.-Z. Zhu, M. Regnier, and M. A. Laflamme. Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells Dev. 22(14):1991–2002, Jul.2013. https://doi.org/10.1089/scd.2012.0490.
Cartledge, J. E., et al. Functional crosstalk between cardiac fibroblasts and adult cardiomyocytes by soluble mediators. Cardiovasc. Res. 105(3):260–270, 2015. https://doi.org/10.1093/cvr/cvu264.
Zhang, X., et al. Cardiomyocyte differentiation induced in cardiac progenitor cells by cardiac fibroblast-conditioned medium. Exp. Biol. Med. (Maywood). 239(5):628–637, 2014. https://doi.org/10.1177/1535370214525323.
Trieschmann, J., et al. The interaction between adult cardiac fibroblasts and embryonic stem cell-derived cardiomyocytes leads to proarrhythmic changes in in vitro cocultures. Stem Cells Int. 2016:2936126, 2016. https://doi.org/10.1155/2016/2936126.
McCurdy, S., C. F. Baicu, S. Heymans, and A. D. Bradshaw. Cardiac extracellular matrix remodeling: fibrillar collagens and secreted protein acidic and rich in cysteine (SPARC). J. Mol. Cell Cardiol. 48(3):544–549, 2010. https://doi.org/10.1016/j.yjmcc.2009.06.018.
Lindsey, M. L., M. Jung, M. E. Hall, and K. Y. DeLeon-Pennell. Proteomic analysis of the cardiac extracellular matrix: clinical research applications. Expert Rev. Proteomics. 15(2):105–112, 2018. https://doi.org/10.1080/14789450.2018.1421947.
Konstandin, M. H., et al. Fibronectin is essential for reparative cardiac progenitor cell response following myocardial infarction. Circ. Res. 2013. https://doi.org/10.1161/CIRCRESAHA.113.301152.
Zhang, J., et al. “Cardiac differentiation of human pluripotent stem cells using defined extracellular matrix proteins reveals essential role of fibronectin. eLife. 11:69028, 2022. https://doi.org/10.7554/eLife.69028.
Stempien, A., et al. Identifying features of cardiac disease phenotypes based on mechanical function in a catecholaminergic polymorphic ventricular tachycardia model. Front. Bioeng. Biotechnol.10:873531, May2022. https://doi.org/10.3389/fbioe.2022.873531.
Acknowledgements
The authors would like to thank Jodi Lawson for her assistance in data processing and manuscript preparation. This research was funded by the University of Wisconsin-Madison, through the Karen Thompson Medhi Professorship, Graduate School, and Office of the Vice Chancellor for Research and Graduate Education (WCC). Support was also provided by the National Institutes of Health, under Ruth L. Kirschstein National Research Service Award T32 HL 007936 from the National Heart Lung and Blood Institute to the University of Wisconsin-Madison Cardiovascular Research Center (AS). TJK and and JZ were supported for this work by National Institutes of Health U01HL134764 and National Science Foundation 1648035. This material is based upon work supported by (while serving at) the National Science Foundation (WCC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health nor the National Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Jane Grande-Allen, PhD oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
13239_2024_711_MOESM1_ESM.tif
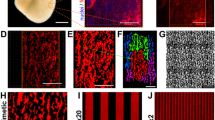
(A) Representative 10x objective images of decellularized ECM used in this study on days 0 (prior to CF seeding on micropatterned substrates), 11. 14, and 18 immunolabeled for Collagen Type I (COL1A; teal) and Laminin (green). Laminin present on day 0 originates from Matrigel patterning. Scale bar = 250 µm. (B) Representative 100x objective images of Laminin remodeling at bridge regions from day 0 to 18. Scale bar = 25 µm. (C) Z – stack thickness of 100x images of decellularized scaffolds on days 0 (Matrigel only), 11, 14, and 18. N = 4 for each day. **p < .01, *** p < .001. Supplementary file9 (TIF 19348 kb)
13239_2024_711_MOESM2_ESM.tif
Heatmap images of the maximum state of contraction for CM Only on Matrigel (top left), CM-CF coculture on Matrigel (top right), CM Only on decellularized ECM (bottom left) and CM-CF coculture on decellularized ECM (bottom right). Maximum states of contraction were identified as the maximum average second principal strain calculated by DIC software. Regions of the imaging field not occupied by cells were excluded from calculations and are denoted by the white regions in the heatmap still. The second principal strain values reported in the legend are in percent. Full length heatmap videos, as well as the corresponding raw phase contrast video for each representative image are included as Supplemental Videos 1-8. Supplementary file10 (TIF 5081 kb)
13239_2024_711_MOESM3_ESM.tif
(Left) From a brightfield video of contracting iPSC-CMs, full-field displacements in x and y and computed for the duration of the video. (Middle) The resulting displacement trajectories are shown. The angle of each vector is computed and small trajectories, likely occurring in the regions where cells are not present, are filtered based on the trajectory magnitudes. (Right) A histogram of the trajectory angles is made, and the primary angle used to compute the percent of all trajectories within 20° of the peak angle. Representative histograms of the trajectory angles for (top) chevron, (middle) lane, and (bottom) monolayer pattern. Supplementary file11 (TIF 1231 kb)
13239_2024_711_MOESM4_ESM.tif
Percent of displacement trajectories with 20° alignment for days 6 (top), 12 (center), and 18 (bottom) in both Matrigel and decellularized ECM and culture conditions on chevron patterned substrates (CM Only = black, CM-CF = red). No significant difference exists in overall alignment on any day. Day 6: N= 25 CM Matrigel, N= 22 CM Decellularized, N= 31 CM-CF Matrigel, N = 22 CM-CF Decellularized. Day 12: N= 25 CM Matrigel, N= 19 CM Decellularized, N= 25 CM-CF Matrigel, N = 19 CM-CF Decellularized. Day 18: N= 18 CM Matrigel, N= 16 CM Decellularized, N= 18 CM-CF Matrigel, N = 19 CM-CF Decellularized. Supplementary file12 (TIF 2734 kb)
13239_2024_711_MOESM5_ESM.tif
Effects of micropatterned lane width on displacement trajectory alignment (A), maximum contractile strain (B), and number of neighboring CMs (C). The number of neighboring CMs was determined as the number of nuclei within 100 μm of each individual nucleus. The threshold of 100 μm was chosen as it is the physiological length of adult CMs. For displacement alignment and strain quantification: N = 26 (20 μm), 27 (40 μm), 31 (60 μm), 32 (80 μm), 32 (100 μm), 32 (120 μm), and 29 (140 μm). For the quantification of neighboring CMs, N = 4 biological replicates, with one image of each lane width of interest or chevron pattern taken from each sample. *p < .05, **p < .01 ***p < .001, two-way ANOVA with post hoc Tukey tests. All experiments were performed with CMs only, in the absence of CFs for the duration of culture. Supplementary file13 (TIF 1838 kb)
13239_2024_711_MOESM6_ESM.docx
(A) Quantification of the percent of myofibrils aligned within 10 degrees of the superior angle for the two culture conditions tested determined by SGFT. N = 8 (4 ROIs within a sample, 2 independent samples). (B) α-actinin expression after 18 days of culture for the CM Only (left) and CM-CF (right). α-actinin = green. Scale bars = 50 µm. Supplementary file14 (DOC 6002 kb)
Representative brightfield video recording of a day 12 CM Only sample on Matrigel. Captured on a Nikon Eclipse Ti microscope with a Plan Flour 10x NA 0.3 objective and Nikon DS-QiMc camera with samples maintained at 37°C. Scale bar = 250 µm. Acquisition rate = 18.92 fps (5.55 s). Supplementary file1 (AVI 26955 kb)
Representative brightfield video recording of a day 12 CM-CF sample on Matrigel. Captured on a Nikon Eclipse Ti microscope with a Plan Flour 10x NA 0.3 objective and Nikon DS-QiMc camera with samples maintained at 37°C. Scale bar = 250 µm. Acquisition rate = 18.92 fps (7.45 s). Supplementary file2 (AVI 36264 kb)
Representative brightfield video recording of a day 12 CM Only sample on decellularized ECM. Captured on a Nikon Eclipse Ti microscope with a Plan Flour 10x NA 0.3 objective and Nikon DS-QiMc camera with samples maintained at 37°C. Scale bar = 250 µm. Acquisition rate = 18.92 fps (8.62 s). Supplementary file3 (AVI 45398 kb)
Representative brightfield video recording of a day 12 CM-CF sample on decellularized ECM. Captured on a Nikon Eclipse Ti microscope with a Plan Flour 10x NA 0.3 objective and Nikon DS-QiMc camera with samples maintained at 37°C. Scale bar = 250 µm. Acquisition rate = 18.92 fps (7.93 s). Supplementary file4 (AVI 43510 kb)
Representative heatmap video of day 12 CM Only sample on Matrigel, corresponding to the Supplementary Video 1. Acquisition rate = 18.92 fps (5.55 s). Supplementary file5 (AVI 19269 kb)
Representative heatmap video of a day 12 CM-CF sample on Matrigel, corresponding to the Supplementary Video 2. Acquisition rate = 18.92 fps (7.45 s). Supplementary file6 (AVI 24307 kb)
Representative heatmap video of a day 12 CM Only sample on decellularized ECM, corresponding to the Supplementary Video 3. Acquisition rate = 18.92 fps (8.62 s). Supplementary file7 (AVI 27644 kb)
Representative heatmap video of a day 12 CM-CF sample on decellularized ECM, corresponding to the Supplementary Video 4. Acquisition rate = 18.92 fps (7.93 s). Supplementary file8 (AVI 24139 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Stempien, A., Josvai, M., Notbohm, J. et al. Influence of Remodeled ECM and Co-culture with iPSC-Derived Cardiac Fibroblasts on the Mechanical Function of Micropatterned iPSC-Derived Cardiomyocytes. Cardiovasc Eng Tech (2024). https://doi.org/10.1007/s13239-024-00711-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13239-024-00711-8