Abstract
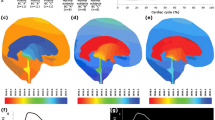
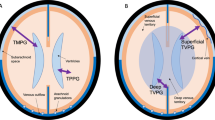
CINE phase-contrast MRI (CINE-MRI) was used to measure cerebrospinal fluid (CSF) velocities and flow rates in the brain of six normal subjects and five patients with communicating hydrocephalus. Mathematical brain models were created using the MRI images of normal subjects and hydrocephalic patients. In our model, the effect of pulsatile vascular expansion is responsible for pulsatile CSF flow between the cranial and the spinal subarachnoidal spaces. Simulation results include intracranial pressure gradients, solid stresses and strains, and fluid velocities throughout the cranio-spinal system. Computed velocities agree closely with our in vivo CINE-MRI CSF flow measurements. In addition to normal intracranial dynamics, our model captures the transition to acute communicating hydrocephalus. By increasing the value for reabsorption resistance in the subarachnoid villi, our model predicts that the poroelastic parenchyma matrix will be drained and the ventricles enlarge despite small transmantle pressure gradients during the transitional phase. The poroelastic simulation thus provides a plausible explanation on how reabsorption changes could be responsible for enlargement of the ventricles without large transmantle pressure gradients.












Similar content being viewed by others
References
Bertram, C.D., A.R., Brodbelt, and M.A. Stoodley. The origins of syringomyelia: numerical models of fluid/structure interactions in the spinal cord. J. Biomech. Eng. 127:1099-1109, 2005. doi:10.1115/1.2073607
Biot, M.A. General theory of three-dimensional consolidation. J. Appl. Phys. 12:155-164, 1941. doi:10.1063/1.1712886
Biot, M.A. Theory of elasticity and consolidation for a porous anisotropic solid. J. Appl. Phys. 26:182-185, 1955. doi:10.1063/1.1721956
Czosnyka, M., Z. Czosnyka, S. Momjian, and J. Pickard. Cerebrospinal fluid dynamics. Physiol. Meas. 25:R51-R76, 2004. doi:10.1088/0967-3334/25/5/R01
Dandy, W. Experimental hydrocephalus. Ann. Surg. 70:129-142, 1919
Fin, L., and R. Grebe. Three dimensional modeling of the cerebrospinal fluid dynamics and brain interactions in the aqueduct of sylvius. Comput. Methods Biomech. Biomed. Eng. 6:163-170, 2003. doi:10.1080/1025584031000097933
Gonzalez-Darder, J.M., and J.L. Barcia-Salorio. Pulse amplitude and volume-pressure relationships in experimental hydrocephalus. Acta Neurochir (Wien). 97:166-170, 1989. doi:10.1007/BF01772830
Greitz, D. Radiological assessment of hydrocephalus: new theories and implications for therapy. Neurosurg. Rev. 27:145-165, 2004
Greitz, D. Unraveling the riddle of syringomyelia. Neurosurg. Rev. 29:251-264, 2006. doi:10.1007/s10143-006-0029-5
Greitz, D., J. Hannerz, T. Rahn, H. Bolander, and A. Ericsson. MR imaging of cerebrospinal fluid dynamics in health and disease. Acta Radiologica. 35:204-211, 1994
Jacobson, E., D. Fletcher, M. Morgan, and I. Johnston. Computer modeling of the cerebrospinal fluid flow dynamics of aqueduct stenosis. Med. Biol. Eng. Comput. 37:59-63, 1999. doi:10.1007/BF02513267
Jacobson, E., D. Fletcher, M. Morgan, and I. Johnston. Fluid dynamics of the cerebral aqueduct. Pediatr. Neurosurg. 24:229-236, 1996. doi:10.1159/000121044
Johanson, C.E., J.A. Duncan III, P.M. Klinge, T. Brinker, E.G. Stopa, and G.D. Silverberg. Multiplicity of cerebrospinal fluid functions: new challenges in health and disease. Cerebrospinal Fluid Res. 5:10, 2008. doi:10.1186/1743-8454-5-10.
Kaczmarek, M., R. Subramaniam, and S. Neff. The hydromechanics of hydrocephalus: steady-state solutions for cylindrical geometry. Bull. Math. Biol. 59:295-323, 1997. doi:10.1007/BF02462005
Linninger, A.A., C. Tsakiris, D.C. Zhu, M. Xenos, P. Roycewicz, Z. Danziger, and R. Penn. Pulsatile cerebrospinal fluid dynamics in the human brain. IEEE Trans. Biomed. Eng. 52:557-565, 2005. doi:10.1109/TBME.2005.844021
Linninger, A.A., M. Xenos, D.C. Zhu, M.B. Somayaji, and R. Penn. Cerebrospinal fluid flow in the normal and hydrocephalic brain. IEEE Trans. Biomed. Eng. 54:291-302, 2007. doi:10.1109/TBME.2006.886853
Linninger, A. A., M. Xenos, B. Sweetman, S. Ponkshe, X. Guo, and R. Penn. A mathematical model of blood, cerebrospinal fluid and brain dynamics. J. Math. Biol., 2009. doi:10.1007/s00285-009-0250-2.
Peña, A., M. Bolton, H. Whitehouse, and J. Pickard. Effects of brain ventricular shape on periventricular biomechanics: a finite-element analysis. Neurosurgery. 45:107, 1999. doi:10.1097/00006123-199907000-00026
Penn, R., and J. Bacus. The brain as a sponge: a computed tomographic look at Hakim’s hypothesis. Neurosurgery. 14:670-675, 1984. doi:10.1097/00006123-198406000-00004
Penn, R., M.C. Lee, A.A. Linninger, K. Miesel, S. Ning Lu, and L. Stylos. Pressure gradients in the brain in an experimental model of hydrocephalus. J. Neurosurg. 102:1069-1075, 2005
Peters, G.P., and D.W. Smith. Solute transport through a deforming porous medium. Int. J. Numer. Anal. Methods Geomech. 26:683-717, 2002. doi:10.1002/nag.219
Pettorossi, V.E., C. Di Rocco, R. Mancinelli, M. Caldarelli, and F. Velardi. Communicating hydrocephalus induced by mechanically increased amplitude of the intraventricular cerebrospinal fluid pulse pressure: rationale and method. Exp. Neurol. 59:30-39, 1978. doi:10.1016/0014-4886(78)90198-X
Smillie, A., I. Sobey, and Z. Molnar. A hydroelastic model of hydrocephalus. J. Fluid Mech. 539:417-443, 2005. doi:10.1017/S0022112005005707
Stephensen, H., M. Tisell, and C. Wikkelso. There is no transmantle pressure gradient in communicating or noncommunicating hydrocephalus. Neurosurgery. 50:763-771, 2002. doi:10.1097/00006123-200204000-00016
Taylor, Z., and K. Miller. Reassessment of brain elasticity for analysis of biomechanisms of hydrocephalus. J. Biomech. 37:1263-1269, 2004. doi:10.1016/j.jbiomech.2003.11.027
Troupp, H. Intracranial pressure in hydrocephalus after subarachnoid hemorrhage. Zentralbl Neurochir. 36:11-17, 1975
Ulug, A., T. Truong, C. Filippi, T. Chun, J. Lee, C. Yang, M. Souweidane, and R. Zimmerman. Diffusion imaging in obstructive hydrocephalus. Am. J. Neuroradiol. 24:1171-1176, 2003
Zhu, D., M. Xenos, A.A. Linninger, and R. Penn. Dynamics of lateral ventricle and cerebrospinal fluid in normal and hydrocephalic brains. J. Magn. Reson. Imaging. 24:756-770, 2006. doi:10.1002/jmri.20679
Zimmerman, R., C. Fleming, B. Lee, L. Saint-Louis, and M. Deck. Periventricular hyperintensity as seen by magnetic resonance: prevalence and significance. Am. J. Roentgenol. 146:443-450, 1986
Acknowledgment
The authors would like to gratefully acknowledge NIH for their partial financial support of this project, NIH-5R21EB004956, as well as a grant from the Stars Kids Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Linninger, A.A., Sweetman, B. & Penn, R. Normal and Hydrocephalic Brain Dynamics: The Role of Reduced Cerebrospinal Fluid Reabsorption in Ventricular Enlargement. Ann Biomed Eng 37, 1434–1447 (2009). https://doi.org/10.1007/s10439-009-9691-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-009-9691-4