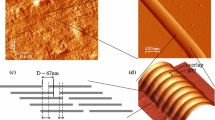
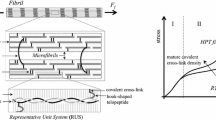
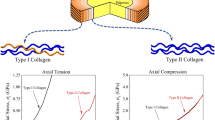
Abstract
The objective of this paper is to investigate the role played by the structural water on the intermolecular sliding between collagen-like 1QSU peptides in a microfibril under deformation. Three modes of deformation are used to generate intermolecular sliding: forced axial stretching (case I) or sliding (case II) of a central peptide monomer (while other surrounding monomers are fixed); and cantilever bending (case III) under a terminal lateral load. The force–displacement curve of each deformation mode is derived using a module called Steered Molecular Dynamics (SMD) in a molecular dynamics package NAMD under the CHARMM22 force field. Each calculation is carried out twice, one in the presence of structural water, one without. It is found that the structural water is a weak “lubricant” in forced axial stretching (case I), but it functions as a “glue” in forced axial sliding (case II) and cantilever bending (case III). A change in the pulling speed does not significantly alter the force–displacement behavior in axial stretching (case I) and sliding (case II), but it does in cantilever bending (case III). The additional resistance contributed by the structural water is attributed to the additional energy cost in breaking the water-mediated hydrogen bonds (water bridges).












Similar content being viewed by others
Abbreviations
- AFM:
-
atomic force microscopy
- BFP:
-
biomembrane force probes
- 1CGD:
-
protein databank ID of the collagen-like, alanine replacement peptide, also called Gly→Ala.
- 1QSU:
-
protein databank ID of the collagen-like, EKG replacement peptide
- BPTI:
-
bovine pancreatic trypsin inhibitor
- CHARMM:
-
Chemistry at HARvard Molecular Mechanics
- CHEM3D:
-
name of a commercial molecular modeling package
- CPU:
-
central processing unit
- EKG:
-
glutamate-lysine-glycine
- GLU:
-
three letter code for glutamate (E)
- GLY:
-
three letter code for glycine (G)
- GROMACS:
-
name of an open-access molecular dynamics package using GROMOS96 force field
- GROMOS96:
-
name of a force field with open access, GROMOS stands for “GROningen MOlecular Simulation”
- LOT:
-
laser optical tweezers
- LYS:
-
three letter code for lysine (K)
- MD:
-
molecular dynamics
- MM:
-
molecular mechanics
- NAMD:
-
Not Another Molecular Dynamics
- NMR:
-
nuclear magnetic resonance
- PDB:
-
protein data bank
- RATTLE:
-
name of an algorithm to apply constraints in MD
- RMSD:
-
root mean square deviation
- SMD:
-
steered molecular dynamics
- SPC/E:
-
name of a configurational model of the water molecule
- TIP3P:
-
name of a configurational model of the water molecule
- VMD:
-
visual molecular dynamics
- WAXD:
-
wide angle X-ray diffraction
- X-PLOR :
-
name of a software used in X-ray crystallography and NMR
References
Andersen H. C. (1983) Rattle: a “velocity” version of the shake algorithm for molecular dynamics calculations. J. Comp. Phys. 52: 24–34
Bailey A. J., Robins S. P., Balian G. (1974) Biological significance of the intermolecular crosslinks of collagen. Nature 251: 105–109
Bella J., Eaton M., Brodsky B., Berman H. M. (1994) Crystal and molecular structure of a collagen-like peptide at 1.9Å resolution. Science 266: 75–81
Bella J., Brodsky B., Berman H. M. (1995) Hydration structure of a collagen peptide. Structure 3: 893–906
Berendsen H. J. C. (1962) Nuclear magnetic resonance study of collagen hydration. J. Chem. Phys. 36(12): 3297–3305
Bohinski R. C. (1987) Modern Concepts in Biochemistry. 5th ed. Englewood Cliffs, New Jersey: Prentice Hall
Broz J. J., Simske S. J., Greenberg A. R., Luttges M. W. (1993) Effects of rehydration state on the flexural properties of whole mouse long bones J. Biomech. Eng. 115(4A): 447–449
Brünger, A. T. X-PLOR, Version 3.1: A System of X-ray Crystallography and NMR. The Howard Hughes Medical Institute and Department of Molecular Biophysics and Biochemistry, Yale University, 1992.
Csermely P. (2001) Water and cellular folding processes Cell Mol. Biol. (Noisy-le-grand). 47(5): 791–800
Currey J. D. (1988) The effects of drying and re-wetting on some mechanical properties of cortical bone. J. Biomech. 21(5): 439–441
Cusack S., Miller A. (1979) Determination of the elastic constants of collagen by Brillouin light scattering J. Mol. Biol. 135: 39–51
Eliav U., Navon G. (2002) Multiple quantum filtered NMR studies of the interaction between collagen and water in the tendon. J. Am. Chem. Soc. 124(12): 3125–3132
Fernandez-Seara M. A., Wehrli S. L., Takahashi M., Wehrli F. W. (2004) Water content measured by proton–deuteron exchange NMR predicts bone mineral density and mechanical properties. J. Bone Miner. Res. 19(2): 289–296
Fischer S., Verma C. S. (1999) Binding of buried structural water increases the flexibility of proteins Proc. Natl. Acad. Sci. USA 96: 9613–9615
Fraser R. D. B., MacRae T. P. (1973) Conformation in Fibrous Proteins. New York, Academic Press
Fraser R. D., MacRae T. P., Miller A., Suzuki E. (1983) Molecular conformation and packing in collagen fibrils. J. Mol. Biol. 167(2): 497–521
Fratzl P., Misof K., Zizak I., Rapp G., Amenitsch H., Bernstorff S. (1998) Fibrillar structure and mechanical properties of collagen J. Struct. Biol. 122: 119–122
Fudge D. S., Gosline J. M. (2004) Molecular design of the α-keratin composite: insights from a matrix-free model, hagfish slime threads. Proc. R. Soc. Lond. B 271: 291–299
Fung Y. C. (1993) Biomechanics: Mechanical Properties of Living Tissues. 2nd Edn. New York, Springer
Glimcher, M. J. Composition, structure, and organization of bone and other mineralized tissues and the mechanism of calcification. In: Handbook of Physiology, vol 7 Endocrinology, edited by R. O. Greep, and E. B. Astwood. Washington, DC: American Physiological Society, 1976
Harley R., James D., Miller A., White J. W. (1977) Phonons and the elastic moduli of collagen and muscle. Nature 267: 285–287
Hofmann H., Voss T., Kuhn K., Engel J. (1984) Localization of flexible sites in thread-like molecules from electron micrographs-Comparison of interstitial, basement membrane and intima collagen. J. Mol. Biol. 172: 325–343
Hopfinger A. J. Intermolecular Interactions and Biomolecular Organization. New York. John Wiley & Sons, pp. 113–143, 1977.
Humphrey W., Dalke A., Schulten K. (1996) VMD: Visual Molecular Dynamics, J. Mol. Graphics 14: 33–38
Hypercube, Inc. Hyperchem 6.2, http://www.hyper.com/products/default.htm (2002)
Isralewitz B, Gao M., Schulten K. (2001) Steered molecular dynamics and mechanical functions of proteins. Curr. Opin. Struct. Biol. 11: 224–230
Izrailev S., Stepaniants S., Balsera M., Oono Y., Schulten K. (1997) Molecular dynamics study of unbinding of the avidin-biotin complex. Biophys. J. 72: 1568–1581
Jarzynski C. (1997) Nonequilibrium equality for free energy differences. Phys. Rev. Lett. 78: 2690–2693
Jorgensen W. L., Chandrasekhar J., Madura J. D., Impey R. W., Klein M. L. (1983) Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79: 926–935
Kramer, R. Z. X-ray crystallographic Structural Studies of Three Collagen-like Peptides, PhD thesis, New Brunswick. New Jersey, USA, Rutgers, SUNJ, 1998.
Kramer R. Z., Berman H. M. (1998) Patterns of hydration in crystalline collagen peptides. J. Biomol. Struct. Dynam. 16: 367–380
Kramer R. Z., Venugopal M., Bella J., Mayville P., Brodsky B., Berman H. M. (2000) Staggered molecular packing in crystals of a collagen-like peptide with a single charged pair. J. Mol. Biol. 301: 1191–1205
Kuznetsova N., Chi S. L., Leikin S. (1998) Sugars and polyols inhibit fibrillogenesis of type I collagen by disrupting hydrogen-bonded water bridges between helices. Biochemistry 37: 11888–11895
Leikin S., Parsegian V. A., Yang W.–H., Walrafen G. E. (1997) Raman spectral evidence for hydration forces between collagen triple helices. Proc. Natl. Acad. Sci. 94: 11312–11317
Lorenzo A. C., Caffarena E. R. (2005) Elastic properties, Young’s modulus determination and structural stability of the tropocollagen molecule: a computational study by steered molecular dynamics. J. Biomech. 38: 1527–1533
Lu H., Isralewitz B., Krammer A., Vogel V., Schulten K. (1998) Unfolding of titin immunoglobulin domains by steered molecular dynamics simulation. Biophys. J. 75: 662–667
Lu H., Schulten K. (2000) The key event in force-induced unfolding of titin’s immunoglobulin domains. Biophys. J. 79: 51–65
MacKerell Jr. A. D., Bashford D., Bellott M., Dunbrack R. L. Jr., Evanseck J. D., Field M. J., Fischer S., Gao J., Guo H., Ha S., Joseph-McCarthy D., Kuchnir L., Kuczera K., Lau F. T. K., Mattos C., Michnick S., Ngo T., Nguyen D. T., Prodhom B., Reiher W. E. III., Roux B., Schlenkrich M., Smith J. C., Stote R., Straub J., Watanabe M., Wiorkiewicz-Kuczera J., Yin D., Karplus M. (1998) All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B. 102: 3586–3616
Makarov V. A., Andrews B. K., Smith P. E., Pettitt B. M. (2000) Residence times of water molecules in the hydration sites of myoglobin. Biophys. J. 79: 2966–2974
Merkel R., Nassoy P., Leung A., Ritchie K., Evans E. (1999) Energy landscapes of receptor-ligand bonds explored with dynamic force spectroscopy. Nature 397: 50–53
Naito A., Tuzi S., Saito H. (1994) A high-resolution 15N solid-state NMR study of collagen and related polypeptides. The effect of hydration on formation of interchain hydrogen bonds as the primary source of stability of the collagen-type triple helix. Eur. J. Biochem. 224: 729–734
Nakasako M. (2004) Water–protein interactions from high-resolution protein crystallography. Phil. Trans. R. Soc. Lond. B 359: 1191–1206
Nestler F. H. M., Hvidt S., Ferry J. D. (1983) Flexibility of collagen determined from dilute solution viscoelastic measurements. Biopolymers 22: 1747–1758
Neuman W. F., Neuman M. W. (1958) The Chemical Dynamics of Bone Mineral. Chicago, IL, University of Chicago Press
Nomura S., Hiltner A., Lando J. B., Baer E. (1977) Interaction of water with native collagen. Biopolymers 16: 231–246
Ottani V., Martini D., Franchi M., Ruggeri A., Raspanti M. (2002) Hierarchical structures in fibrillar collagens. Micron 33: 587–596
Paci E., Karplus M. (1999) Forced unfolding of fibronectin type 3 modules: An analysis by biased molecular dynamics simulations. J. Mol. Biol. 288: 441–459
Petruska J. A., Hodge A. J. (1964) A subunit model for the tropocollagen macromolecule. Proc. Natl. Acad. Sci. USA 51: 871–876
Phillips J. C., Braun R., Wang W., Gumbart J., Tajkhorshid E., Villa E., Chipot C., Skeel R. D., Kale L., Schulten K. (2005) Scalable molecular dynamics with NAMD. J. Comput. Chem. 26: 1781–1802
Piekarski K. (1973) Analysis of bone as a composite material Int. J. Eng. Sci. 11(6A): 557
Pineri M. H., Escoubes M., Roche G. (1978) Water-collagen interactions: Calorimetric and mechanical experiments. Biopolymers 17: 2799–2815
Robinson R. A. (1952) An electron microscopic study of the crystalline inorganic component of bone and its relationship to the organic matrix. J. Bone Joint Surg. 34-A: 389–434
Sasaki N., Odajima S. (1996) Stress–strain curve and Young’s modulus of a collagen molecule as determined by the X-ray diffraction technique. J. Biomech. 29(5): 655–658
Smith J. W. (1968) Molecular pattern in native collagen. Nature 219: 157–158
Thornton G. M., Shrive N. G., Frank C. B. (2001) Altering ligament water content affects ligament pre-stress and creep behavior. J. Orthop. Res. 19: 845–851
Vesentini S., Fitie C. F. C., Montevecchi F. M., Redaelli A. (2005) Molecular assessment of the elastic properties of collagen-like homotrimer sequences. Biomechan. Model. Mechanobiol. 3: 224–234
Williams R. J. P., Fausto da Silva J. J. R. (1996) The Natural Selection of the Chemical Elements. New York, Oxford University Press
Yeni Y. N., Brown C. U., Norman T. L. (1998) Influence of bone composition and apparent density on fracture toughness of the human femur and tibia. Bone 22(1): 79–84
Acknowledgment
The authors acknowledge the financial supports from Rutgers, State University of New Jersey. The authors also thank Virginia Dare for proofreading a final version of this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, D., Chippada, U. & Jordan, K. Effect of the Structural Water on the Mechanical Properties of Collagen-like Microfibrils: A Molecular Dynamics Study. Ann Biomed Eng 35, 1216–1230 (2007). https://doi.org/10.1007/s10439-007-9296-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-007-9296-8