Abstract
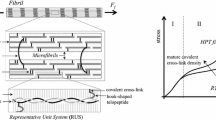
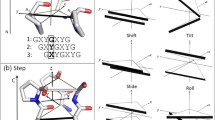
Knowledge of the mechanical behavior of collagen molecules is critical for understanding the mechanical properties of collagen fibrils that constitute the main architectural building block of a number of connective tissues. In this study, the elastic properties of four different type I collagen 30-residue long molecular sequences, were studied by performing stretching simulations using the molecular mechanics approach. The energy–molecular length relationship was achieved by means of the geometry optimization procedure for collagen molecule strains up to 10%. The energy was interpolated by a second order function, and the second order of the derivative with respect to the mean length corresponded to the molecule stiffness. According to the hypothesis of linear elastic behavior, except for one sequence, the elastic modulus was around 2.40 GPa. These values are larger than fibril values, and they confirm the hypothesis that tendon mechanical properties are deeply related to tendon hierarchical structure. A possible explanation of the lowest values obtained for one sequence (1.33–1.53 GPa) is provided and discussed.






Similar content being viewed by others
References
Beck K, Brodsky B (1998) Supercoiled protein motifs: the collagen triple-helix and the alpha-helical coiled coil. J Struct Biol 122:17–29
Bella J, Eaton M, Brodsky B, Berman HM, (1994) Crystal and molecular structure of a collagen-like peptide at 1.9 Å resolution. Science 266:75–81
Berisio R, Vitagliano L, Mazzarella L, Zagari A (2000–2001) Crystal structure of a collagen-like polypeptide with repeating sequence Pro-Hyp-Gly at 1.4 A resolution: implications for collagen hydration. Biopolymers 56:8–13
Berisio R, Vitagliano L, Mozzarella L, Zagari A, (2002) Crystal structure of the collagen triple helix model [(Pro-Pro-Gly)10]3. Protein Sci 11:262–270
Berisio R, Granata V, Vitagliano L, Zagari A (2004). Characterization of collagen-like heterotrimers: implications for triple-helix stability. Biopolymers 73:682–688
Brodsky B, Ramshaw JA, (1997) The collagen triple-helix structure. Matrix Biol 15:545–554
Cusack S, Miller A (1979) Determination of the elastic constants of collagen by Brillouin light scattering. J Mol Biol 135:39–51
Folkhard W, Mosler E, Geercken E, Knorzer H, Nemetschek-Gansler H, Nemetschek T (1987) Quantitative analysis of the molecular sliding mechanism in native tendon collagen-time resolved dynamic studies using synchrotron radiation. Int J Biol Macromol 9:169–175
Fratzl P, Misof K, Zizak I, Rapp G, Amenitsch H, Bernstorff S (1997) Fibrillar structure and mechanical properties of collagen. J Struct Biol 122:119–122
Harley R, James D, Miller A, White JW (1977) Phonons and the elastic moduli of collagen and muscle. Nature 267:285–287
Hofmann H, Fietzek PP, Kuhn K (1980) Comparative analysis of the sequences of the three collagen chains α1(I), α2 and α 1(III). J Mol Biol 141:293–314
Hofmann H, Voss T, Kuhn K, Engel J (1984) Localization of flexible sites in thread-like molecules from electron micrographs. Comparison of interstitial, basement membrane and intima collagens. J Mol Biol 172:325–343
Hulmes DJS, Miller A, Parry DAD, Piez KA, Woodhead-Galloway J (1973) Analysis of the primary structure of collagen for the origins of molecular packing . J Mol Biol 79:137–148
Jimenez SA, Bashey RI, Benditt M, Yankowski R (1977) Identification of collagen alpha1(I) trimer in embryonic chick tendons and calvaria. Biochem Biophys Res Commun 78:1354–1361
Keene DR, San Antonio JD, Mayne R, McQuillan DJ, Sarris G, Santoro SA, Iozzo RV (2000) Decorin Binds Near the C Terminus of Type I Collagen. J Biol Chem 276:21801–21804
Kielty CM, Hopkinson I, Grant ME (1993) In: Royce PM, Steinmann B (eds) Connective tissue its heritable disorders. Wiley, New York, pp 103–148
Kramer RZ, Venugopal MG, Bella J, Mayville P, Brodsky B, Berman HM (2000) Staggered molecular packing in crystals of a collagen-like peptide with a single charged pair. J Mol Biol 301:1191–1205
Kramer RZ, Bella J, Brodsky B, Berman HM (2001). The crystal and molecular structure of a collagen-like peptide with a biologically relevant sequence. J Mol Biol 311:131–147
Kuivaniemi H, Tromp G, Prockop DJ (1997) Mutations in fibrillar collagens (types I, II, III, and XI), fibril-associated collagen (type IX), and network-forming collagen (type X) cause a spectrum of diseases of bone, cartilage, and blood vessels. Hum Mutat 9:300–315
Landau LD, Lifshitz EM (1986) Theory of elasticity. Pergamon, Oxford
Landau LD, Lifshitz EM (1970) Statistical physics. Pergamon, Oxford
McBride DJ Jr, Choe V, Shapiro JR, Brodsky B (1997) Altered collagen structure in mouse tail tendon lacking the alpha 2(I) chain. J Mol Biol 270:275–284
Morawetz H (1975) Chapter V and VI. In: Wiley J (ed) Macromolecules in solution. Wiley, New York
Mosler E, Folkhard W, Knorzer E, Nemetschek-Gansler H, Nemetschek T, Koch MHJ (1985) Stress-induced molecular rearrangement in tendon collagen. J Mol Biol 182:589–596
Okuyama K, Arnott S, Takayanagi M, Kakudo M (1981) Crystal and molecular structure of a collagen-like polypeptide (Pro-Pro-Gly)10. J Mol Biol 152:427–443
Orgel JP, Wess TJ, Miller A (2000) The in situ conformation and axial location of the intermolecular cross-linked non-helical telopeptides of type I collagen. Structure 8:137–142
Ottani V, Martini D, Franchi M, Ruggeri A, Raspanti M (2002) Hierarchical structures in fibrillar collagens. Micron 33:587–596
Persikov AV, Ramshaw JA, Brodsky B (2000) Collagen model peptides: Sequence dependence of triple-helix stability. Biopolymers 55:436–450
Prockop DJ, Fertala A (1998) The collagen fibril: the almost crystalline structure. J Struct Biol 122:111–118
Rainey JK, Goh MC (2004b) An interactive triple-helical collagen builder. Bioinformatics 20:2458–2459
Rainey JK, Goh MC, (2004b) Statistically based reduced representation of amino acid side chains. J Chem Inf Comput Sci 44:817–830
Ramshaw JA, Shah NK, Brodsky B (1998) Gly-X-Y tripeptide frequencies in collagen: a context for host-guest triple-helical peptides. J Struct Biol 122:86–91
Raspanti M, Alessandrini A, Ottani V, Ruggeri A (1997) Direct visualization of collagen-bound proteoglycans by tapping-mode atomic force microscopy. J Struct Biol 119:118–122
Raspanti M, Congiu T, Alessandrini A, Gobbi P, Ruggeri A (2000) Different patterns of collagen-proteoglycan interaction: a scanning electron microscopy and atomic force microscopy study. Eur J Histochem 44:335–343
Raspanti M, Congiu T, Guizzardi S (2002) Structural aspects of the extracellular matrix of tendon: an atomic force and scanning electron microscopy study. Arch Histol Cytol 65:37–43
Redaelli A, Vesentini S, Soncini M, Vena P, Mantero S, Montevecchi FM (2003) The possible role of decorin glycosaminoglycans in fibril to fibril force transfer in relative mature tendons—a computational study from molecular to microstructural level. J Biomech 36:1555–1569
Rich A, Crick EHC (1961) The molecular structure of collagen. J Mol Biol 3:483–506
Sasaki N, Odajima S (1996) Stress–strain curve and Young’s modulus of a collagen molecule as determined by the X-ray diffraction technique. J Biomech 29:655–658
Scott JE (1988) Proteoglycan-fibrillar collagen interactions. Biochem J 252:313–323
Scott JE (1992) Supramolecular organization of extracellular matrix glycosaminoglycans, in vitro and in the tissues. FASEB J 6:2639–2645
Silver FH, Horvath I, Foran DJ. (2002) Mechanical implications of the domain structure of fiber-forming collagens: comparison of the molecular and fibrillar flexibilities of the alpha1-chains found in types I–III collagen. J Theor Biol 216:243–254
Sun YL, Luo ZP, Fertala A, An KN (2002) Direct quantification of the flexibility of type I collagen monomer. Biochem Biophys Res Commun 295:382–386
Uitto J (1979) Collagen polymorphism: isolation and partial characterization of alpha 1(I)-trimer molecules in normal human skin. Arch Biochem Biophys 192:371–379
Vitagliano L, Nemethy G, Zagari A, Scherana HA (1995) Structure of the type I collagen molecule based on conformational energy computations: the triple-stranded helix and the N-terminal telopeptide. J Mol Biol 247:69–80
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vesentini, S., Fitié, C.F.C., Montevecchi, F.M. et al. Molecular assessment of the elastic properties of collagen-like homotrimer sequences. Biomech Model Mechanobiol 3, 224–234 (2005). https://doi.org/10.1007/s10237-004-0064-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-004-0064-5