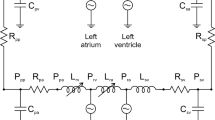
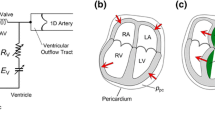
Abstract
While numerous computer models exist for the circulatory system, many are limited in scope, contain unwanted features or incorporate complex components specific to unique experimental situations. Our purpose was to develop a basic, yet multifaceted, computer model of the left heart and systemic circulation in LabVIEW™ having universal appeal without sacrificing crucial physiologic features. The program we developed employs Windkessel-type impedance models in several open-loop configurations and a closed-loop model coupling a lumped impedance and ventricular pressure source. The open-loop impedance models demonstrate afterload effects on arbitrary aortic pressure/flow inputs. The closed-loop model catalogs the major circulatory waveforms with changes in afterload, preload, and left heart properties. Our model provides an avenue for expanding the use of the ventricular equations through closed-loop coupling that includes a basic coronary circuit. Tested values used for the afterload components and the effects of afterload parameter changes on various waveforms are consistent with published data. We conclude that this model offers the ability to alter several circulatory factors and digitally catalog the most salient features of the pressure/flow waveforms employing a user-friendly platform. These features make the model a useful instructional tool for students as well as a simple experimental tool for cardiovascular research.
Similar content being viewed by others
References
Alexander, J., K. Sunagawa, N. Chang, and K. Sagawa. Instantaneous pressure–volume relation of the ejecting canine left atrium. Circ. Res. 61(2):209–219, 1987.
Arnold, J. M. O., G. E. Marchiori, J. R. Imrie, G. L. Burton, P. W. Pflugfelder, and W. J. Kostuk. Large artery function in patients with chronic heart failure. Circulation 84:2418–2425, 1991.
Burkhoff, D., D. Kass, W. L. Maughan, and A. Kolandaivelu. CVSIM v1.5. http://oac.med.jhmi.edu/cvsim/; 1998.
Chen, C.-W., Y.-W. R. Shau, and C.-P. Wu. Analog transmission line model for simulation of systemic circulation. IEEE Trans. Biomed. Eng. 44(1):90–94, 1997.
Chung, D. C., S. C. Niranjan, J. W. Clark, Jr., A. Bidani, W. E. Johnston, J. B. Zwischenberger, and D. L. Traber. A dynamic model of ventricular interaction and pericardial influence. Am. J. Physiol. 272:H2942–H2962, 1997 (Heart Circ. Physiol. 41).
Danielsen, M. Modeling of feedback mechanisms which control the heart function in a view to an implementation in cardiovascular models. In: Mathematics, 2nd ed. Roskilde: Roskilde University, 1998, p. 145.
Danielsen, M., and J. T. Ottesen. Describing the pumping heart as a pressure source. J. Theor. Biol. 212:71–81, 2001.
Danielsen, M., J. L. Palladino, and A. Noordergraaf. The left ventricular ejection effect. In: Mathematical Modeling in Medicine, edited by J. T. Ottesen and M. Danielsen. Netherlands: IOS Press, 2000, pp. 13–28.
Dankelman, J., H. G. Stassen, and J. A. E. Spaan. Coronary circulation mechanics. In: Coronary Circulation: Basic Mechanism and Clinical Relevance, edited by F. Kajiya, G. A. Klassen, J. A. E. Spaan, and J. I. E. Hoffman, New York: Springer-Verlag, 1990, pp. 75–87.
Davis, M. J., and R. W. Gore. Determinants of cardiac function: simulation of a dynamic cardiac pump for physiology instruction. Adv. Physiol. Educ. 25(1):13–35, 2001.
Elzinga, G., and N. Westerhof. Pressure and flow generated by the left ventricle against different impedances. Circ. Res. 32(2):178–186, 1973.
Ferrari, G., M. Kozarski, C. De Lazzari, F. Clemente, M. Merolli, G. Tosti, M. Guaragno, R. Mimmo, D. Ambrosi, and J. Glapinski. A hybrid (numerical-physical) model of the left ventricle. Int. J. Artif. Organs 24(7):456–462, 2001.
Finkelstein, S. M., J. N. Cohn, V. R. Collins, P. F. Carlyle, and W. J. Shelley. Vascular hemodynamic impedance in congestive heart failure. Am. J. Cardiol. 55:423–427, 1985.
Goerke, J. Jon Goerke's Arterial Simulation. http:// hemodynamics.ucdavis.edu/.
Grossman, W., E. Braunwald, and T. Mann. Contractile state of the left ventricle in man as evaluated from end-systolic pressure–volume relations. Circulation 56(5):845–852, 1977.
Guyton, A. C., and J. E. Hall. Textbook of Medical Physiology, 10th ed. Philadelphia: Saunders, 2000.
Hoffman, J. I. E. Pressure–flow relationships of the coronary arteries. In: Coronary Circulation: Basic Mechanisms and Clinical Relevance, edited by F. Kajiya, G. A. Klassen, J. A. E. Spaan, and J. I. E. Hoffman. New York: Springer-Verlag, 1990, pp. 109–125.
Kleinbaum, D. G., and L. L. Kupper. Applied Regression Analysis and Other Multivariable Methods. North Scituate, MA: Duxbury Press, Division of Wadsworth Publishing Company Inc., 1978.
Laskey, W. K., and W. G. Kussmaul. Arterial wave reflection in heart failure. Circulation 75(4):711–722, 1987.
Liu, Z., F. Shen, and F. C. P. Yin. Impedance of arterial system simulated by viscoelastic t tubes terminated in windkessels. Am. J. Physiol. 256(4 Pt 2):H1087–H1099, 1989.
Lu, K., J. W. Clark, Jr., F. H. Ghorbel, C. S. Robertson, D. L. Ware, J. B. Zwischenberger, and A. Bidani. Cerebral autoregulation and gas exchange studied using a human cardiopulmonary model. Am. J. Physiol. 286:H584–H601, 2003 (Heart Circ. Physiol.).
Lucas, C. L. Fluid mechanics of the pulmonary circulation. CRC Crit. Rev. Biomed. Eng. 10(4):317–392, 1984.
Mantero, S., R. Pietrabissa, and R. Fumero. The coronary bed and its role in the cardiovascular system: A review and an introductory single-branch model. J. Biomed. Eng. 14:109–116, 1992.
Maruyama, Y., and T. Takishima. Effect of ventricular and extraventricular pressure on the coronary artery pressure–flow relationship. In: Coronary Circulation: Basic Mechanisms and Clinical Relevance, edited by F. Kajiya, G. A. Klassen, J. A. E. Spaan, and J. I. E. Hoffman. New York: Springer-Verlag, 1990, pp. 127–138.
Maughan, W. L., and K. Sunagawa. Factors affecting the end-systolic pressure–volume relationship. Fed. Proc. 43(9):2408–2410, 1984.
Maughan, W. L., K. Sunagawa, D. Burkhoff, and K. Sagawa. Effect of arterial impedance changes on end-systolic pressure–volume relation. Circ. Res. 54(5):595–602, 1984.
Mulier, J. P. Ventricular pressure as a function of volume and flow. University of Leuven, Belgium, 1994.
Murgo, J. P., N. Westerhof, J. P. Giolma, and S. A. Altobelli. Aortic input impedance in normal man: Relationship to pressure wave forms. Circulation 62(1):105–116, 1980.
Murgo, J. P., N. Westerhof, J. P. Giolma, and S. A. Altobelli. Effects of exercise on aortic input impedance and pressure wave forms in normal humans. Circ. Res. 48(3):334–343, 1981.
Nichols, W. W., C. R. Conti, W. E. Walker, and W. R. Milnor. Input impedance of the systemic circulation in man. Circ. Res. 40(5):451–458, 1977.
Nichols, W. W., M. F. O'Rourke, and C. Hartley. McDonald's blood flow in arteries: Theoretical, experimental and clinical principles, 4th ed. London: Arnold, 1998.
Nishioka, O., Y. Maruyama, K. Ashikawa, S. Isoyama, S. Satoh, J. Watanabe, Y. Shimizu, and T. Takishima. Load dependency of end-systolic pressure–volume relations in isolated, ejecting canine hearts. Jpn. Heart J. 29(5):709–722, 1988.
Noble, M. I. Left ventricular load, arterial impedance and their interrelationship. Cardiovasc. Res. 13(4):183–198, 1979.
O'Rourke, M. F. Arterial Function in Health and Disease. Edinburgh, NY: Churchill Livingstone, 1982.
Olansen, J. B., J. W. Clark, D. Khoury, F. Ghorbel, and A. Bidani. A closed-loop model of the canine cardiovascular system that includes ventricular interaction. Comput. Biomed. Res. 33:260–295, 2000.
Ottesen, J. T. Modelling of the baroreflex-feedback mechanism with time-delay. J. Math. Biol. 36:41–63, 1997.
Ottesen, J. T. Nonlinearity of baroreceptor nerves. Surv. Math. Ind. 7:187–201, 1997.
Ottesen, J. T. Modelling the dynamical baroreflex-feedback control. Math. Comp. Model. 31:167–173, 2000.
Ottesen, J. T., and M. Danielsen. Modeling ventricular contraction with heart rate changes. J. Theor. Biol. 222:337–346, 2003.
Ottesen, J. T., M. S. Olufsen, and J. K. Larsen. Applied Mathematical Models in Human Physiology. Philadelphia: SIAM, 2004.
Palladino, J. L., G. M. Drzewiecki, and A. Noordergraaf. Modeling strategies in physiology. In: The Biomedical Engineering Handbook, edited by J. D. Bronzino. Boca Raton, FL: CRC Press, IEEE Press, 1995, pp. 2367–2374.
Palladino, J. L., J. P. Mulier, and A. Noordergraaf. Closed-loop circulation model based on the Frank mechanism. Surv. Math. Ind. 7:177–186, 1997.
Palladino, J. L., J. P. Mulier, F. Wu, M. Moser, T. Kenner, R. M. Baevsky, and A. Noordergraaf. Assessing the state of the circulatory system via parameters vs. variables. J. Cardiovasc. Diagnosis Procedures 13(2):131–139, 1996.
Palladino, J. L., and A. Noordergraaf. The changing view of the heart through the centuries. Stud. Health Technol. Inform. 71:3–11, 2000.
Palladino, J. L., L. C. Ribeiro, and A. Noordergraaf. Human circulatory system model based on Frank's mechanism. In: Mathematical Modeling in Medicine, edited by J. T. Ottesen and M. Danielsen. Thes Netherlands: IOS Press, 2000, pp. 29–40.
Paulus, W. J., V. A. Claes, and D. L. Brutsaert. End-systolic pressure–volume relation estimated from physiologically loaded cat papillary muscle contractions. Circ. Res. 47(1):20–26, 1980.
Pepine, C. J., W. W. Nichols, and C. R. Conti. Aortic input impedance in heart failure. Circulation 58(3):460–465, 1978.
Pietrabissa, R., S. Mantero, T. Marotta, and L. Menicanti. A lumped parameter model to evaluate the fluid dynamics of different coronary bypasses. Med. Eng. Phys. 18:477–484, 1996.
Rideout, V. C. Mathematical and Computer Modeling of Physiological Systems. Madison, WI: Medical Physics Publishing, 1991.
Rose, W. C., and A. A. Shoukas. Two-port analysis of systemic venous and arterial impedances. Am. J. Physiol. 265:H1577–H1587, 1993 (Heart Circ. Physiol. 34).
Sagawa, K. The ventricular pressure–volume diagram revisited. Circ. Res. 43(5):677–687, 1978.
Sagawa, K. The end-systolic pressure–volume relation of the ventricle: Definition, modifications and clinical use. Circulation 63(6):1223–1227, 1981.
Sagawa, K., L. Maughan, H. Suga, and K. Sunagawa. Cardiac Contraction and the Pressure–Volume Relationship. New York: Oxford University Press, 1988.
Stergiopulos, N., B. E. Westerhof, and N. Westerhof. Total arterial inertance as the fourth element of the windkessel model. Am. J. Physiol. 276(1 Pt 2):H81–H88, 1999.
Suga, H., and K. Sagawa. Instantaneous pressure–volume relationships and their ratio in the excised, supported canine left ventricle. Circ. Res. 35(1):117–126, 1974.
Sunagawa, K., W. L. Maughan, G. Friesinger, P. Guzman, M.-S. Chang, and K. Sagawa. Effects of coronary arterial pressure on left ventricular end-systolic pressure–volume relation of isolated canine heart. Circ. Res. 50(5):727–734, 1982.
Sunagawa, K., K. Sagawa, and W. L. Maughan. Ventricular interaction with the loading system. Ann. Biomed. Eng. 12(2):163–189, 1984.
Timmons, W. D. Cardiovascular models and control. In: The Biomedical Engineering Handbook, edited by J. D. Bronzino. Boca Raton, FL: CRC Press, IEEE Press, 1995, pp. 2386–2403.
Tsitlik, J. E., H. R. Halperin, A. S. Popel, A. A. Shoukas, F. C. P. Yin, and N. Westerhof. Modeling the circulation with three-terminal electrical networks containing special non-linear capacitors. Ann. Biomed. Eng. 20:595–616, 1992.
Tsujioka, K., M. Goto, O. Hiramatsu, Y. Wada, Y. Ogasawara, and F. Kajiya. Functional characteristics of intramyocardial capacitance vessels and their effects on coronary arterial inflow and venous outflow. In: Coronary Circulation: Basic Mechanisms and Clinical Relevance, edited by F. Kajiya, G. A. Klassen, J. A. E. Spaan, and J. I. E. Hoffman. New York: Springer-Verlag, 1990, pp. 89–97.
Ursino, M. Interaction between carotid baroregulation and the pulsating heart: A mathematical model. Am. J. Physiol. 275:H1733–H1747, 1998 (Heart Circ. Physiol. 44).
Ursino, M., M. Antonucci, and E. Belardinelli. Role of active changes in venous capacity by the carotid baroreflex: Analysis with a mathematical model. Am. J. Physiol. 267:H2531–H2546, 1994 (Heart Circ. Physiol. 36).
Watanabe, I., T. A. Johnson, C. L. Engle, C. Graebner, M. G. Jenkins, and L. S. Gettes. Effects of Verapamil and Propranolol on changes in extracellular K +, pH, and local activation during graded coronary flow in the pig. Circulation 79(4):939–947, 1989.
Watt, T. B., and C. S. Burrus. Arterial pressure contour analysis for estimating human vascular properties. J. Appl. Physiol. 40(2):171–176, 1976.
Westerhof, N. Arterial haemodynamics. In: The Physics of Heart and Circulation, edited by J. Strackee and N. Westerhof. Philadelphia: Institute of Physics Pub., 1993, pp. 355–381.
Westerhof, N., and G. Elzinga. Normalized input impedance and arterial decay time over heart period are independent of animal size. Am. J. Physiol. 261(1 Pt 2):R126–R133, 1991.
Westerhof, N., G. Elzinga, and S. Pieter. An artificial system for pumping hearts. J. Appl. Physiol. 31(5):776–781, 1971.
Zamir, M. The Physics of Pulsatile Flow. New York: Springer-Verlag, 2000.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cole, R.T., Lucas, C.L., Cascio, W.E. et al. A LabVIEW™ Model Incorporating an Open-Loop Arterial Impedance and a Closed-Loop Circulatory System. Ann Biomed Eng 33, 1555–1573 (2005). https://doi.org/10.1007/s10439-005-7785-1
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10439-005-7785-1