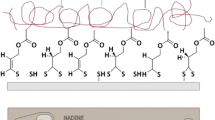
Abstract
Microchip electrophoresis in a poly(dimethylsiloxane) microfluidic device is one of the versatile separation techniques in a micro total analysis system, while an unstable electroosmotic flow depending on an inner surface of a microchannel and a nonspecific adsorption of biogenic compounds onto the inner surface are often problematic in microchip electrophoresis. To overcome these drawbacks, a chitin-coated poly(dimethylsiloxane) microchannel was newly developed by a relatively simple experimental procedure based on vacuum drying. The obtained chitin-coating showed high durability during 10-times experiments with a stable electroosmotic flow. It was also confirmed that the chitin-coated poly(dimethylsiloxane) microchannel provided the pH-dependent electroosmotic flow related to the dissociations of amino and silanol groups of chitin and poly(dimethylsiloxane), respectively. The nonspecific adsorption of fluorescently labeled proteins onto the inner surface of the channel was well suppressed by the coating, resulting in the sharp and symmetric peak without tailing in the microchip electrophoretic analysis of proteins. The separation of the proteins was also demonstrated in the chitin-coated microchannel, so that the resolution and reproducibility of the migration time were improved as compared to those in the untreated microchannel.






Similar content being viewed by others
References
Belder D, Ludwig M (2003) Surface modification in microchip electrophoresis. Electrophoresis 24:3595–3606
Chen L, Ren J, Bi R, Chen D (2004) Ultraviolet sealing and poly(dimethylacrylamide) modification for poly(dimethylsiloxane)/glass microchips. Electrophoresis 25:914–921
Chen Y, Zhang L, Chen G (2008) Fabrication, modification, and application of poly(methyl methacrylate) microfluidic chips. Electrophoresis 29:1801–1814
Dittrich PS, Tachikawa K, Manz A (2006) Micro total analysis systems. Latest advancements and trends. Anal. Chem 78:3887–3907
Dou YH, Bao N, Xu JJ, Meng F, Chen HY (2004) Separation of proteins on surface-modified poly(dimethylsiloxane) microfluidic devices. Electrophoresis 25:3024–3031
Duffy DC, McDonald JC, Schueller OJA, Whitesides GM (1998) Rapid prototyping of microfluidic systems in poly(dimethylsiloxane). Anal Chem 70:4974–4984
Effenhauser CS, Manz A, Widmer HM (1993) Glass chips for high-speed capillary electrophoresis separations with submicrometer plate heights. Anal Chem 65:2637–2642
Effenhauser CS, Bruin GJM, Paulus A, Ehrat M (1997) Integrated capillary electrophoresis on flexible silicone microdevices: analysis of DNA restriction fragments and detection of single DNA molecules on microchips. Anal Chem 69:3451–3457
Foster RE, Hert DG, Chiesl TN, Fredlake CP, Barron AE (2009) DNA migration mechanism analyses for applications in capillary and microchip electrophoresis. Electrophoresis 30:2014–2024
Harrison DJ, Fan Z, Seiler K, Manz A, Widmer HM (1993) Rapid separation of fluorescein derivatives using a micromachined capillary electrophoresis system. Anal Chem Act 283:361–366
Hruška V, Jaroš M, Gaš B (2006) Simul 5—free dynamic simulator of electrophoresis. Electrophoresis 27:984–991
Jayakumar R, Selvamurugan N, Nair SV, Tokura S, Tamura H (1999) Preparative methods of phosphorylated chitin and chitosan—an overview. Int J Biol Macromol 20:180–188
Kawai T, Sueyoshi K, Kitagawa F, Otsuka K (2010) Microchip electrophoresis of oligosaccharides using large-volume sample stacking with electroosmotic flow pump in single channel. Anal Chem 82:6504–6511
Kenyon SM, Meighan MM, Hayes MA (2011) Recent developments in electrophoretic separations on microfluidic devices. Electrophoresis 32:482–493
Kitagawa F, Kubota K, Sueyoshi K, Otsuka K (2006) One-step immobilization of cationic polymer onto a poly(methyl methacrylate) microchip for high performance electrophoretic analysis of proteins. Sci Technol Adv Mat 7:558–565
Kitagawa F, Kubota K, Sueyoshi K, Otsuka K (2010) One-step preparation of amino-PEG modified poly(methyl methacrylate) microchips for electrophoretic separation of biomolecules. J Pharm Biomed Anal 53:1272–1277
Liang RP, Gan GH, Qiu JD (2008) Surface modification of poly(dimethylsiloxane) microfluidic devices and its application in simultaneous analysis of uric acid and ascorbic acid in human urine. J Sep Sci 31:2860–2867
Liu J, Lee ML (2006) Permanent surface modification of polymeric capillary electrophoresis microchips for protein and peptide analysis. Electrophoresis 27:3533–3546
Liu Y, Fanguy JC, Bledsoe JM, Henry CS (2000) Dynamic coating using polyelectrolyte multilayers for chemical control of electroosmotic flow in capillary electrophoresis microchips. Anal Chem 72:5939–5944
Liu Y, Fu X, Bai Y, Zhai M, Liao Y, Liao J, Liu H (2011) Improvement of reproducibility and sensitivity of CE analysis by using the capillary coated dynamically with carboxymethyl chitosan. Anal Bioanal Chem 399:2821–2829
Makamba H, Kim JH, Lim K, Park N, Hahn JH (2003) Surface modification of poly(dimethylsiloxane) microchannels. Electrophoresis 24:3607–3619
Manz A, Graber N, Widmer HM (1990) Miniaturized total chemical analysis systems: a novel concept for chemical sensing. Sensor Actuat B-Chem 1:244–248
Manz A, Harrison DJ, Verpoorte E, Fettinger JC, Paulus A, Lüdi H, Widmer HM (1992) J Chromatogr A 593:253–258
Muck A, Svatoš A (2007) Chemical modification of polymeric microchip devices. Talanta 74:333–341
Ocvirk G, Munroe M, Tang T, Oleschuk R, Westra K, Harrison DJ (2000) Electrokinetic control of fluid flow in native poly(dimethylsiloxane) capillary electrophoresis devices. Electrophoresis 21:107–115
Pallandre A, de Lambert B, Attia R, Jonas AM, Viovy JL (2006) Surface treatment and characterization: perspectives to electrophoresis and lab-on-chips. Electrophoresis 27:584–610
Peng Y, Pallandre A, Tran NT, Taverna M (2008) Recent innovations in protein separation on microchips by electrophoretic methods. Electrophoresis 29:157–178
Phillips KS, Kottegoda S, Kang KM, Sims CE, Allbritton NL (2008) Separations in poly(dimethylsiloxane) microchips coated with supported bilayer membranes. Anal Chem 80:9756–9762
Qiu J, Hu P, Liang R (2007) Separation and simultaneous determination of uric acid and ascorbic acid on a dynamically modified poly(dimethylsiloxane) microchip. Anal Sci 23:1409–1414
Ro KW, Lim K, Kim H, Hahn JH (2002) Poly(dimethylsiloxane) microchip for precolumn reaction and micellar electrokinetic chromatography of biogenic amines. Electrophoresis 23:1129–1137
Rogers CI, Pagaduan JV, Nordin GP, Woolley AT (2011) Single-monomer formulation of polymerized polyethylene glycol diacrylate as a nonadsorptive material for microfluidics. Anal Chem 83:6418–6425
Sikanen T, Tuomikoski S, Ketola RA, Kostiainen R, Franssila S, Kotiaho T (2007) Fully microfabricated and integrated su-8-based capillary electrophoresis-electrospray ionization microchips for mass spectrometry. Anal Chem 79:9135–9144
Soper SA, Ford SM, Qi S, McCarley RL, Kelly K, Murphy MC (2000) Polymeric microelectromechanical systems. Anal Chem 72:643A–651A
Sueyoshi K, Nagai H, Wakida S, Nishii J, Kitagawa F, Otsuka K (2006) Application of partial filling technique to electrophoretic analysis on microchip with T-cross channel configuration. Meas Sci Technol 17:3154–3161
Sueyoshi K, Kitagawa F, Otsuka K (2008) On-Line sample preconcentration and separation technique based on transient trapping in microchip micellar electrokinetic chromatography. Anal Chem 80:1255–1262
Tamura H, Nagahama H, Tokura S (2006) Preparation of chitin hydrogel under mild conditions. Cellulose 13:357–364
Tran NT, Ayed I, Pallandre A, Taverna M (2010) Recent innovations in protein separation on microchips by electrophoretic methods: an update. Electrophoresis 31:147–173
Wang AJ, Xu JJ, Zhang Q, Chen HY (2006a) The use of poly(dimethylsiloxane) surface modification with gold nanoparticles for the microchip electrophoresis. Talanta 69:210–215
Wang AJ, Xu JJ, Chen HY (2006b) Proteins modification of poly(dimethylsiloxane) microfluidic channels for the enhanced microchip electrophoresis. J Chromatogr A 1107:257–264
Wang AJ, Xu JJ, Chen HYJ (2007) In situ grafting hydrophilic polymer on chitosan modified poly(dimethylsiloxane) microchip for separation of biomolecules. J Chromatogr A 1147:120–126
West J, Becker M, Tombrink S, Manz A (2008) Micro total analysis systems: latest achievements. Anal Chem 80:4403–4419
Woolley AT, Mathies RA (1995) Ultra-high-speed DNA sequencing using capillary electrophoresis chips. Anal Chem 67:3676–3680
Wu D, Luo Y, Zhou X, Dai Z, Lin B (2005) Multilayer poly(vinyl alcohol)-adsorbed coating on poly(dimethylsiloxane) microfluidic chips for biopolymer separation. Electrophoresis 26:211–218
Wu D, Qin J, Lin B (2008) Electrophoretic separations on microfluidic chips. J Chromatogr A 1184:542–549
Young EWK, Berthier E, Guckenberger DJ, Sackmann E, Lamers C, Meyvantsson I, Huttenlocher A, Beebe DJ (2011) Rapid prototyping of arrayed microfluidic systems in polystyrene for cell-based assays. Anal Chem 83:1408–1417
Zhang QL, Xu JJ, Lian HZ, Li XY, Chen HY (2007) Polycation coating poly(dimethylsiloxane) capillary electrophoresis microchip for rapid separation of ascorbic acid and uric acid. Anal Bioanal Chem 387:2699–2704
Zhou J, Ellis AV, Voelcker NH (2010) Recent developments in PDMS surface modification for microfluidic devices. Electrophoresis 31:2–16
Acknowledgments
K.S. is grateful to the Grant-in-Aid for Young Scientists (B) (22750070) from the Japan Society for the Promotion of Science (JSPS), Grant-in-Aid for the Global COE Program from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan, and the Shimadzu Science Foundation. A part of this development was made by the SENTAN from the Japan Science and Technology Agency (JST).
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix: Normalization of the values of electroosmotic mobility
In the experiment, the values of electroosmotic mobility (μeo) were estimated by employing the untreated/chitin-coated microchannels filled with the various background solutions (BGSs). It is well-known that a value of μeo depends on both the pH and ionic strength (I) of the BGS. Thus, the experimentally obtained values of μeo under the different BGS conditions should be normalized by the ionic strength to evaluate the dependence of the μeo on the pH of the BGS. Generally, the value of μeo is linear to the value of (I)−1/2. Hence, the values of I in the different BGS was calculated by using Simul 5 software [Hruška et al. 2006]. The compositions and pH of the BGS, values of I, (I)−1/2, μeo and standardized μeo (μeo,st) are summarized in Tables A1 and A2.
Contact angle measurement
To evaluate the effect of the chitin-coating on the hydrophobicity/hydrophilicity of the surface of a poly(dimethylsiloxane) (PDMS) substrate, contact angles of a water droplet on the untreated, O2 plasma-treated, Ca solvent-treated, and chitin-coated PDMS planer plates were measured. Preparation procedures were described as follows:
A large planar PDMS plate (circular shape; diameter, 4 inch) was made by the conventional procedure. It was then cut as 1-inch square pieces. Some of them were dipped into a Ca solvent (CaCl2 2H2O-saturated methanol, see main manuscript) or chitin solution prepared with the Ca solvent and then dried under a vacuum condition. Some of untreated PDMS plates were treated with the O2 plasma for activation of the surface.
As a result of the contact angle measurement, the obtained values were summarized in Table A3. Regarding the hydrophobicity of the surface, chitin-coating reduced the contact angle from 108º on the untreated one to 95º, whereas the Ca solvent-treatment did not affect. In the case of O2 plasma treatment which is need for bonding of the PDMS plates, contact angle was drastically decreased by the activation of silanol group as well-known. However, it was also well-known that the activated silanol groups were gradually deactivated, so that the contact angle on O2 plasma-treated surface was increased from 38º to 61º and 94 º after 30 min and 1 day, respectively. This variation of the surface characteristic directly causes the unstable electroosmotic flow in the PDMS microchannel, which is inferior to microchip electrophoresis.
Rights and permissions
About this article
Cite this article
Sueyoshi, K., Hori, Y. & Otsuka, K. Inner surface modification of poly(dimethylsiloxane) microchannel with chitin for electrophoretic analysis of proteins. Microfluid Nanofluid 14, 933–941 (2013). https://doi.org/10.1007/s10404-012-1100-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10404-012-1100-x