Abstract
Purpose
To compare the efficacy and safety of a combination therapy of prednisolone and cyclosporine and corticosteroid pulse therapy in Vogt–Koyanagi–Harada (VKH) disease.
Study design
A prospective, multicenter, randomized, non-inferiority trial.
Methods
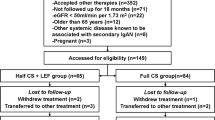
Patients of new-onset acute VKH disease at 11 centers in Japan between 2014 and 2018 were randomized to a combination (oral prednisolone 60 mg daily with gradual taper-off to 35 mg/day and cyclosporine 3 mg/kg/day) and corticosteroid (methylprednisolone 1000 mg for 3 days followed by oral prednisolone) groups, and were followed for 1 year.
Results
Thirty-four were assigned to the combination and thirty-six patients to the corticosteroid group. Recurrence/worsening risk was 0.15 (95% confidence-interval [CI] 0.03–0.27) in the combination group and 0.25 (95% CI 0.11–0.39) in the corticosteroid group, with a risk difference of − 0.10 (90% CI − 0.27 to 0.06), demonstrating non-inferiority of the combination group with a non-inferiority margin of 0.20 (P = 0.0013). Serious adverse events occurred in three patients (two with hyponatremia and one with severe headaches) in the combination group and none in the corticosteroid group. Sunset glow fundus grades and cataract rates at 1 year were 0.57 (95% CI 0.42–71) and 4.3% in the combination group and 0.91 (95% CI 0.78–1.04) and 34.0% in the corticosteroid group, respectively.
Conclusions
Combination therapy was noninferior to corticosteroid therapy with respect to recurrence/worsening risk. Notably, the recurrence/worsening risk, sunset glow fundus grade, and cataract rate were lower in the combination group than in the corticosteroid group.






Similar content being viewed by others
References
Sugiura S. Some observations on uveitis in Japan, with special reference to Vogt–Koyanagi–Harada and Behcet diseases. Nippon Ganka Gakkai Zasshi. 1976;80:1285–326 ((In Japanese)).
Moorthy RS, Inomata H, Rao NA. Vogt–Koyanagi–Harada syndrome. Surv Ophthalmol. 1995;39:265–92.
Read RW, Holland GN, Rao NA, Tabbara KF, Ohno S, Arellanes-Garcia L, et al. Revised diagnostic criteria for Vogt–Koyanagi–Harada disease: report of an international committee on nomenclature. Am J Ophthalmol. 2001;131:647–52.
Yamaki K, Gocho K, Hayakawa K, Kondo I, Sakuragi S. Tyrosinase family proteins are antigens specific to Vogt–Koyanagi–Harada disease. J Immunol. 2000;165:7323–9.
Sugita S, Sagawa K, Mochizuki M, Shichijo S, Itoh K. Melanocyte lysis by cytotoxic T lymphocytes recognizing the MART-1 melanoma antigen in HLA-A2 patients with Vogt–Koyanagi–Harada disease. Int Immunol. 1996;8:799–803.
Sugita S, Takase H, Taguchi C, Imai Y, Kamoi K, Kawaguchi T, et al. Ocular infiltrating CD4+ T cells from patients with Vogt–Koyanagi–Harada disease recognize human melanocyte antigens. Investig Ophthalmol Vis Sci. 2006;47:2547–54.
Lambe T, Leung JC, Bouriez-Jones T, Bouriez-Jonew T, Silver K, Makinen K, et al. CD4 T cell-dependent autoimmunity against a melanocyte neoantigen induces spontaneous vitiligo and depends upon Fas-Fas ligand interactions. J Immunol. 2006;177:3055–62.
Sonoda K-H, Hasegawa E, Namba K, Okada AA, Ohguro N, Goto H, et al. Epidemiology of uveitis in Japan: a 2016 retrospective nationwide survey. Jpn J Ophthalmol. 2021;65:184–90.
Islam SM, Numaga J, Fujino Y, Hirata R, Matsuki K, Maeda H, et al. HLA class II genes in Vogt–Koyanagi–Harada disease. Investig Ophthalmol Vis Sci. 1994;35:3890–6.
Shindo Y, Inoko H, Yamamoto T, Ohno S. HLA-DRB1 typing of Vogt–Koyanagi–Harada’s disease by PCR-RFLP and the strong association with DRB1*0405 and DR*0410. Br J Ophthalmol. 1994;78:223–6.
Damico FM, Kiss S, Young LH. Vogt–Koyanagi–Harada disease. Semin Ophthalmol. 2005;20:183–90.
Inoue R, Taguchi C, Kawahara S, Yamakawa R. A fifteen-year review of Vogt–Koyanagi–Harada disease. Jpn J Clin Ophthalmol. 2011;65:1431–4 (in Japanese).
Iwahashi C, Okuno K, Hashida N, Nakai K, Ohguro N, Nishida K. Incidence and clinical features of recurrent Vogt–Koyanagi–Harada disease in Japanese individuals. Jpn J Ophthalmol. 2015;59:157–63.
Rubsamen PE, Gass JD. Vogt–Koyanagi–Harada syndrome: clinical course, therapy, and longterm visual outcome. Arch Ophthalmol. 1991;109:682–7.
Al-Kharashi AS, Aldibhi H, Al-Fraykh H, Kangave D, Abu El-Asrar AM. Prognostic factors in Vogt–Koyanagi–Harada disease. Int Ophthalmol. 2007;27:201–10.
Lai TYY, Chan RPS, Chan CKM, Lam DSC. Effects of the duration of initial oral corticosteroid treatment on the recurrence of inflammation in Vogt–Koyanagi–Harada disease. Eye. 2009;23:543–8.
Kotake S, Ohno S. “Pulse” methylprednisolone therapy in the treatment of Vogt–Koyanagi–Harada disease. Jpn J Clin Ophthalmol. 1984;38:1053–8 (in Japanese).
Yamanaka E, Ohguro N, Yamamoto S, Nakagawa Y, Imoto Y, Tano Y. Evaluation of pulse corticosteroid therapy for Vogt–Koyanagi–Harada disease assessed by optical coherence tomography. Am J Ophthalmol. 2002;134:454–6.
Hirooka K, Saito W, Namba K, Mizuuchi K, Iwada D, Hashimoto Y, et al. Early post-treatment choroidal thickness to alter sunset glow fundus in patients with Vogt–Koyanagi–Harada disease treated with systemic corticosteroids. PLoS One. 2017;12:e0172612.
Nakayama M, Keino H, Watanabe T, Okada AY. Clinical features and visual outcomes of 111 patients with new-onset acute Vogt–Koyanagi–Harada disease treated with pulse intravenous corticosteroids. Br J Ophthalmol. 2019;103:274–8.
Nussenblatt RB, Palestine AG, Chan CC. Cyclosporin A therapy in the treatment of intraocular inflammatory disease resistant to systemic corticosteroids and cytotoxic agents. Am J Ophthalmol. 1983;96:275–82.
Paredes I, Ahmed M, Foster CS. Immunomodulatory therapy for Vogt–Koyanagi–Harada patients as first-line therapy. Ocul Immunol Inflamm. 2006;14:87–90.
Urzua CA, Velasquez V, Sabat P, Berger O, Ramirez S, Goecke A, et al. Earlier immunomodulatory treatment is associated with better visual outcomes in a subset of patients with Vogt–Koyanagi–Harada disease. Acta Ophthalmol. 2015;93:e475–80.
Oo EEL, Chee S-P, Wong KKY, Htoon HM. Vogt–Koyanagi–Harada disease managed with immunomodulatory therapy within 3 months of disease onset. Am J Ophthalmol. 2020;220:37–44.
Keino H, Goto H, Usui M. Sunset glow fundus in Vogt–Koyanagi–Harada disease with or without chronic ocular inflammation. Graefes Arch Clin Exp Ophthalmol. 2002;240:878–82.
Keino H, Goto H, Mori H, Iwasaki T, Usui M. Association between severity of inflammation in CNS and development of sunset glow fundus in Vogt–Koyanagi–Harada disease. Am J Ophthalmol. 2006;141:1140–1.
Chee SP, Jap A, Bacsal K. Spectrum of Vogt–Koyanagi–Harada disease in Singapore. Int Ophthalmol. 2007;27:137–42.
Tugal-Tutkun I, Ozyazgan Y, Akova YA, Sullu Y, Akyol N, Soylu M, et al. The spectrum of Vogt–Koyanagi–Harada disease in Turkey. Int Ophthalmol. 2007;27:117–23.
Sakata VM, da Silva FT, Hirata CE, Marin MLC, Rodrigues H, Kalil J, et al. High rate of clinical recurrence in patients with Vogt–Koyanagi–Harada disease treated with early high-dose corticosteroids. Graefes Arch Clin Exp Ophthalmol. 2015;253:785–90.
Watanabe N, Sakamoto K, Taniguchi K, Kondoh Y, Kimura T, Kataoka K, et al. Efficacy of combined therapy with cyclosporin and low-dose of prednisolone in interstitial pneumonia associated with connective tissue disease. Clin Investig. 2014;87:467–77.
The Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–16.
Miyanaga M, Kawaguchi T, Shimizu K, Miyata K, Mochizuki M. Influence of early cerebrospinal fluid-guided diagnosis and early high-dose corticosteroid therapy on ocular outcomes of Vogt–Koyanagi–Harada disease. Int Ophthalmol. 2007;27:183–8.
Jaffe GJ, Dick AD, Brezin AP, Nguyen QD, Thorne JE, Kestelyn P, et al. Adalimumab in patients with active noninfectious uveitis. N Engl J Med. 2016;375:932–43.
Nguyen QD, Merrill PT, Jaffe G, Dick AD, Kurup SK, Sheppard J, et al. Adalimumab for prevention of uveitic flare in patients with inactive noninfectious uveitis controlled by corticosteroids (VISUAL II): a multi-center, double-masked, randomized, placebo-controlled phase 3 trial. Lancet. 2016;388:1183–92.
Suhler EB, Adan A, Brezin A, Fortic E, Goto H, Jaffe G, et al. Safety and efficacy of adalimumab in patients with noninfectious uveitis in an ongoing open-label study: VISUAL III. Ophthalmology. 2018;125:1075–87.
de Kort EA, de Lil HS, Bremmers MEJ, Bremmers ME, van Groningen LFJ, Blijlevens NMA, et al. Cyclosporine A trough concentrations are associated with acute GvHD after non-myeloablative allogeneic hematopoietic cell transplantation. PLoS One. 2019;14:e0213913.
Takubo M, Tanaka S, Kushimoto K, Ikeda J, Ogawa K, Suzuki Y, et al. Hyponatremia associated with prophylactic low-dose trimethoprim during systemic corticosteroid therapy for AQP4-Positive optic neuritis in a diabetic patient. Antibiotics. 2020;9:201.
Abu El-Asrar AM, Dosari M, Hemachandran S, Gikandi PW, Al-Muammar A. Mycophenolate mofetil combined with systemic corticosteroids prevents progression to chronic recurrent inflammation and development of “sunset glow fundus” in initial-onset acute uveitis associated with Vogt–Koyanagi–Harada disease. Acta Ophthalmol. 2017;95:85–90.
Papasavvas I, Tugal-Tutlun I, Herbort CP. Vogt–Koyanagi–Harada is a curable autoimmune disease: early diagnosis and immediate dual steroidal and non-steroidal immunosuppression are crucial prerequisites. J Curr Ophthalmol. 2020;32:310–4.
Acknowledgements
A list of Study Group Members and Safety and Efficacy Monitoring Committee Members are Miyata Eye Hospital, Miyakonojo, Japan: Manabu Mochizuki, MD, PhD, Takashi Ono, MD, Takashi Komizo, MD, Kazurori Miyata, MD, PhD; Department of Ophthalmology, Tokyo Medical University, Tokyo, Japan: Hiroshi Goto, MD, PhD, Akihiko Umazume, MD, PhD, Yoshihiko Usui, MD, PhD; Department of Ophthalmology, Jikei University School of Medicine, Tokyo, Japan: Tsutomu Sakai, MD, PhD, Hideo Kohno, MD, Ranko Aoyagi, MD; Department of Ophthalmology and Visual Science, Tohoku University Graduate School of Medicine, Sendai, Japan: Fumihiko Nitta, MD, PhD, Kazuichi Maruyama, MD, PhD; Department of Ophthalmology, Yokohama City University School of Medicine, Yokohama, Japan: Nobuhisa Mizuki, MD, PhD, Etsuko Shibuya, MD, Yasutsugu Ida, MD; Department of Ophthalmology & Visual Science, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University, Tokyo, Japan: Hiroshi Takase, MD, PhD, Shintaro Horie, MD, PhD, Mitsunao Ide, MD, Yuko Iwasaki, MD, PhD, Koju Kamoi, MD, PhD, Yukiko Terada, MD; Department of Ophthalmology and Visual Sciences, Yamagata University Faculty of Medicine, Yamagata, Japan: Yutaka Kaneko, MD, PhD; Department of Ophthalmology, Nippon Medical School, Tokyo, Japan: Junko Hori, MD, PhD, Tomoko Yui, MD, Ayaka Takeda, MD; Department of Ophthalmology, Oita University, Faculty of Medicine, Oita, Japan: Satoko Nakano, MD, PhD; Department of Ophthalmology, Faculty of Medicine, University of Miyazaki, Miyazaki, Japan: Nobuhisa Nao-I, MD, PhD; Department of Ophthalmology, Japan Community Health Care Organization Osaka Hospital, Osaka, Japan: Nobuyuki Ohguro, MD, PhD; School of Data Science, Yokohama City University, Kanagawa, Japan: Makoto Tomita, PhD; Department of Ophthalmology, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan: Ko-hei Sonoda, MD, PhD; Department of Ophthalmology, Japan Community Health Care Organization Tokyo Shinjuku Medical Center, Tokyo, Japan: Yujiro Fujino, MD, PhD; Biostatistical Research Corporation, Tokyo, Japan: Toshihito Furukawa, PhD; Department of Renal Transplantation, Masuko Memorial Hospital, Nagoya, Japan: Kunio Morozumi, MD, PhD. And also a list of Study Group Members and Safety and Efficacy Monitoring Committee Members is available online.
The authors are grateful for the support of the Safety and Efficacy Monitoring Committee members, including Koh-Hei Sonoda, M.D., PhD. (Kyushu University); Yujiro Fujino, M.D., Ph.D. (Japan Community Health Care Organization Tokyo Shinjuku Medical Center); Toshihito Furukawa, Ph.D. (Biostatistical Research Corporation); and Kunio Morozumi, M.D., Ph.D. (Masuko Memorial Hospital).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
T. Ono, Lecture fee (Bayer, Santen, AbbVie); H. Goto, None; T. Sakai, None; F. Nitta, None; N. Mizuki, None; H. Takase, Lecture fee (Santen, Novartis, AbbVie, Senju, Eisai, Otsuka); Y. Kaneko, None; J. Hori, None; S. Nakano, Grant (Novartis); N. Nao-I, None; N. Ohguro, None; K. Miyata, Grant, Lecture fee (AMO, Alcon, Senju, Santen), Grant (Kissei, Chugai, Bayer, M's science, Novartis), Lecture fee (Otsuka, Kowa, HOYA, J-TEC, JFC Sales Plan); M. Tomita, None; M. Mochizuki, None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding Author: Manabu Mochizuki
A list of Study Group Members and Safety and Efficacy Monitoring Committee Members is available both in Acknowledgements and online.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Ono, T., Goto, H., Sakai, T. et al. Comparison of combination therapy of prednisolone and cyclosporine with corticosteroid pulse therapy in Vogt–Koyanagi–Harada disease. Jpn J Ophthalmol 66, 119–129 (2022). https://doi.org/10.1007/s10384-021-00878-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-021-00878-w