Abstract
The nature of ionic liquids (ILs) facilitates their analysis by ion chromatography which, unlike conventional high-performance liquid chromatography, enables analysis both of cations and anions. This paper describes a pioneering ion-chromatographic investigation of IL cations and statistical evaluation of quantitative structure–retention relationships with the objective of predicting the molecular mechanism responsible for retention. Eleven ionic liquid imidazolium and pyridinium cations were analyzed on a CS15 cation-exchange column by isocratic elution with acetonitrile–methanesulfonic acid mixtures. Structural descriptors of the cations obtained from molecular modeling were used to describe their hydrophobicity as determined by chromatography. The most statistically significant were three-term QSRR regression equations relating log k w to analyte n-octanol–water partition coefficient (log P), dipole moment (μ), solvent accessible surface area (A SAS), and hydration energy (HE). They indicate the important role of both hydrophobic and polar the retention of ILs on the CS15 column.
Similar content being viewed by others
Introduction
Ionic liquids (ILs) are salts comprising molecular ions, organic cations, and organic or inorganic anions. Frequently used cations are pyridinium or imidazolium derivatives, depicted in Fig. 1; tetrafluoroborate, hexafluorophosphate, chloride, and bromide are frequently used as inorganic counterions. The IL term is generally associated with salts in which the ions are poorly coordinated and thus have relatively low melting points (<100 °C). ILs have unusual physical and chemical properties, because of the combination of cations and anions; these are non-volatility, a non-flammability, excellent chemical and thermal stability, and selective solubility. Such properties make them essential in biotechnological applications and in the pharmaceutical and chemical industry [1–4].
Despite the ionic character of ILs, separation and analysis of their cations reported so far are generally based on high-performance liquid chromatographic methods employing a variety of column packings and mobile phases [5, 6]. Ion chromatography (IC) is a new approach to analysis of ILs, but few papers on this subject have been published [7–11]. According to the results presented in all of these, IC is an appropriate technique and useful method for analysis of chloride, bromide or iodine impurities in ionic liquids and for quantitative analysis of IL cations [7–11]. The newest approach in the field of IL analysis by IC is simultaneous analysis of cations and anions. A tandem of columns containing cation and anion exchangers followed by conductometric detection has been used successfully [11]. IC can be used not only for analysis of IL cations but also to furnish information about retention mechanism, and to enable prediction of retention by use of quantitative structure–retention relationships (QSRRs).
The QSRRs, developed by Kaliszan [12], are statistically derived relationships between chromatographic properties and descriptors characterizing the molecular structure of the analytes. A typical QSRR study comprises the following steps:
-
1
acquisition of the experimental data set;
-
2
molecular structure entry;
-
3
structure descriptor calculation; and
-
4
regression analysis relating the experimental data with the structural descriptors.
Usually, these relationships are developed by software and are based on the linear or multilinear regression methods. The simplest QSRR model eded for retention prediction is represented by Eq. 1:
where k w is retention factor extrapolated to a pure water as mobile phase; k 1 and k 2 are regression coefficients, characteristic of the separation systems used, representing differences in individual physicochemical properties between the mobile and the stationary phases; and log P is the logarithm of n-octanol–water partition coefficient which can serve as a measure of hydrophobicity [13, 14]. Eq. 1 may be, however, applied only of compounds with very similar structure and the same functional groups.
This model can be used for chromatographic determination of log P. For ILs there are few reports of estimation of this property by the shake flask method. The pioneering work on partition of ILs between n-octanol and water was that of Kaar et al. [15]. In later studies the same method was used for imidazolium cations [16–18]. log P values increase with increasing alkyl chain length of the cation and increasing ILs concentration. The shake flask experimental method is, however, poorly reproducible, and for ILs is also complicated because of their dissociation [14]. For this reason new methods are required for measurement or calculation of log P. One of these utilizes of QSRR equations. There is one report of the use of QSRR for determination of the hydrophobicity of ILs by reversed-phase high-performance liquid chromatography [19]. There are, however, no reports of the use of IC.
In this investigation, we used the IC of IL cations with QSRR to predict the main interactions responsible for retention of the analytes. Eleven IL imidazolium and pyridinium cations were analyzed by use of a cation-exchange column and isocratic elution with acetonitrile–methanesulfonic acid mixtures. Retention data for the IL cations were used to derive QSRR for logarithms of retention factors normalized to a hypothetical zero percent organic modifier mobile phase treated as a dependent variable. This value was also compared with log P and structural data calculated for IL cations.
Experimental
Materials and Reagents
Ionic liquids selected for the investigation were obtained from Merck (Darmstadt, Germany) and from Professor B. Jastorff’s collection (University of Bremen, Germany) synthesized in the laboratory of Professor B. Ondruschka (University of Jena, Germany). Their names, molecular formulas, and main properties are listed in Table 1.
Isocratic-grade acetonitrile (J.T. Baker, Deventer, The Netherlands), methanesulfonic acid (MSA; Fluka, Buchs, Switzerland) for ion chromatography, and deionized water prepared with a Milli-Q purification system (Millipore, Bedford, MA, USA) were used for mobile phase preparation. The pH of the MSA was measured before mixing with the organic modifier. The potassium hydroxide for suppressor regeneration was obtained from POCh (Gliwice, Poland).
Instrumentation
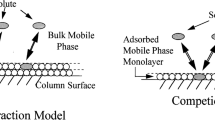
A Dionex model ICS-3000 ion chromatograph consisting of an autosampler, a degasser, pumps, a thermostat, a conductometric detector, and a personal computer with Chromeleon 6.8 software was used for all the chromatographic measurement of the retention data of IL cations (Dionex Corporation, Sunnyvale, CA, USA). The Dionex micromembrane suppressor CMMS 300 (4 mm) for chemical suppression was installed between the conductometric detector and the Dionex IonPac CS15 analytical column (4 × 250 mm, 8.5 μm). The column consists of a macroporous polymer of ethylvinylbenzene crosslinked with 55% divinylbenzene and functionalized with carboxyl and phosphonate cation-exchange sites and crown-ether groups. The crown ethers are attached covalently to the core whereas the phosphonic and the carboxyl groups are connected to aliphatic chains, which are themselves bonded covalently to the core. These chains can represent “tentacles” on which the carboxyl and phosphonate groups have been placed (Fig. 2) [20]. A Dionex IonPac CG15 (4 × 50 mm) guard column was also used. pH was measured with a CP-505 (Elmetron, Zabrze, Poland) pH meter. PTFE membrane filters (0.2 μm; BGB Analytik, Boeckten, Switzerland) were used for filtration.
Methods
Chromatographic Procedure
The analytes were eluted by isocratic elution with acetonitrile–MSA mixtures at a flow rate of 1 mL min−1. Both mobile phase components were chosen as recommended by the manufacturer for the CS15 column [20]. Acetonitrile is the only organic solvent up to 100% compatible with this column. The concentration of the ILs was 0.1 mM in aqueous solution and the injection volume was 20 μL. Each analysis was performed in three replication. During chromatographic investigations both the guard and the analytical columns were thermostated at 35 °C, and the suppressor and conductometric detector at 30 °C. Those temperatures were chosen because of the specification and manufacturer recommendation. The micromembrane suppressor was continuously regenerated by use of 40 mM KOH solution at a flow rate of 7 mL min−1.
The concentration of MSA in the mobile phase varied from 1 to 9 mM and in the volume concentration of acetonitrile from 20 to 75% (v/v). The column hold-up volume, V M, necessary for calculation of retention factors k = V R/V M − 1, was measured by use of the mobile phase system peak.
QSRR Procedure
The retention factors of the ILs were used for determination of log k w, by use of the Snyder–Soczewinski equation (Eq. 2) of the linear solvent strength (LSS) model [21]:
where k is the retention factor, the meaning of k w is the same as in in Eq. 1, S is a constant for a given analyte or congeneric group of analytes, and φ is the volume fraction of the organic component of the mobile phase.
All the structural descriptors were derived by use of HyperChem software with the ChemPlus extension (Hypercube, Waterloo, Canada). They are summarized in Table 1 and were obtained after modeling of the structures of the molecules and optimization their geometry. The calculation procedure used was the semi-empirical quantum-chemical AM1 method. All calculations were performed under simulated vacuum conditions, as this is most typical for the HyperChem software. For the QSRR analysis the multiple regression procedure was executed and QSRR equations derived in this study contained the following molecular structural descriptors of the analytes: logarithm of the n-octanol–water partition coefficient (log P), solvent (water) accessible surface area (A SAS), total dipole moment (μ) polarizability (PO), hydration energy (HE), and total energy (TE).
Statistical evaluation was performed on a personal computer with the Statistica package (StatSoft, Tulsa, USA) and graphs were plotted by use of OriginPro software (OriginLab, MA, USA).
Results and Discussion
IC Analysis of ILs
The k values measured for all mobile phase compositions are summarized in Table 2. Figure 3 shows a typical chromatogram obtained from separation of IL cations with 20% (v/v) acetonitrile and 5 mM MSA. In general, as acetonitrile concentration increases from 20 to 60%, retention decreases. IL had the same retention behavior as on the reversed-phase stationary phases commonly used in HPLC. If up to 65% of acetonitrile was used the IL cations were eluted on the basis of their hydrophobicity (Tables 1, 2). This can be justified by the structure of the packing material used in the investigation. On the surface of the stationary phase there are short alkyl chains, crown ether groups, and cation-exchange sites. It seems that the mechanism of retention of IL cations is predominantly dependent not only on electrostatic forces but also on hydrophobic interactions between the non-polar parts of the stationary phase surface, i.e. the crown-ether and aliphatic chains, with the alkyl chains of the imidazolium and pyridinium cations.
The opposite retention behavior occurred when acetonitrile was used in excess of 65% (v/v). The analytes showed normal phase HPLC retention behavior and the most hydrophobic cation OMIM was eluted before the hydrophilic EEIM (Table 2). This behavior was observed only for use of 1 mM MSA, because of its low eluent strength.
Nevertheless, in some cases similar k were observed for IL cations of different hydrophobicity, i.e., having different log P values (Tables 1, 2). The reason for this effect was explained in detail in our earlier study [22].
Elution of IL cations is, however, affected by both the amount of acetonitrile and the concentration of MSA. k values are reduced when the concentration of MSA is increased.
Chromatographic Behavior of ILs in the IC system
Chromatographic retention of ILs may be explained by another phenomenon. It has been proved that ILs form heterogeneous solutions or ion pairs when they are dissolved in water or other solvents [23, 24]. When ILs enter the chromatographic system they become dissociated salts. Therefore, IL cation and anion can form ion-pairs with ions of the mobile phase (in our case MSA). Although this effect seems to have a rather slight effect on IL retention behavior (because the concentrations and volumes of IL used are low) it is worth discussing.
Ruiz-Angel et al. [24] showed that, depending on the type of salt used as mobile phase component, retention of ILs increased with increasing affinity of the inorganic anions for the apolar stationary phases (chaotropicity). Retention of an imidazolium or pyridinium cation may be a consequence of hydrophobic interactions of the ion-pair, and ion-exchange with the anion-covered stationary phase surface or with the cation exchange sites [23, 24].
The conditions used in our study also promote ion pair formation between IL cations or anions and MSA. First MSA anions can be adsorbed by the stationary phase surface and change its properties [24]. BF4 is an chaotropic anion, which may be adsorbed by the packing material more strongly than the MSA anion [24]. Therefore, the observed retention differences for high or low concentrations of MSA are meaningful. For concentrations >2.5 mM, MSA forms ion pairs with imidazolium and pyridinium cations, and BF4 covers the surface of the packing. The retention of IL cations will be mainly differentiated by their hydrophobicity. On the other hand reducing the amount of MSA (1 mM) in the mobile phase will, consequently, lead to a change in the mechanism of retention. BF4 anions must form ion-pairs with IL cations, because the quantity of MSA anions has been reduced. IL cations are then retained inside the column, mostly by cation-exchange and polar interactions.
Determination of log k w by IC
The retention times of all the IL cations eluted isocratically by acetonitrile–MSA mixtures were used for estimation of log k w of the Snyder–Soczewinski equation (Eq. 2) (Table 2). log k w characterizes the relative hydrophobicity of ILs and can serve as an alternative to the log P.
Hydrophobicity is important because of its effect on most of the important processes occurring in biological systems [25]. The shake flask experimental method, normally used for log P determination is time-consuming, expensive, poorly reproducible, and not suitable for ILs, because of their ionic character [14]. Therefore, other methods are needed for measuring the hydrophobicity of ILs. One of the possibilities are calculation methods. However, they are also more difficult for ionic compounds than for non-polar molecules, especially with independent atom coefficients or the presence of charge [26]. Also the manner in which a positive charge is delocalized in the IL cation is still not fully understood and is left for manual estimation [27].
The log P values calculated by use of HyperChem software are presented in Table 1. The calculation process performed with this software is based on an atom fragment method developed by Ghose et al. [28]. log P is estimated as a sum of atom-based fragment values and the charge of the analyzed cations is not considered. The molecules are treated as neutral species, therefore this method does not seem to be precise for ionic substances, especially ILs.
A more accurate method is estimation of log P for IL cations by other experimental technique, for example IC, used for the first time in this work. This technique enables determination of hydrophobicity in a more complex manner, because the log k w value includes not only the ability to participate in hydrophobic interactions but also the ion-exchange potential of IL cations.
Considering that five different concentration of MSA were used, five values of log k w were obtained for each IL cation. However, all the values of log k w correlate significantly with each other as shown in the Table 3. Therefore, for further QSRR studies the log k w corresponding to the retention factor in pure 7.5 mM MSA were selected, because these had the highest correlation coefficients between log k and φ. Table 4 summarizes regression data for determination of log k w of the Snyder–Soczewinski equation (Eq. 2). This value increases with increasing molar mass for a series of imidazolium ILs and for arylimidazolium and pyridinium cations. Nevertheless, concluding that log k w increase with increasing molecular mass for all ILs is wrong. The observed trend is valid only for series of cations with the same essential building units.
The highest correlation between log k and φ (Table 4) was achieved for IL cations of the highest hydrophobicity (Table 1). This effect is indicative of different retention mechanisms for polar and hydrophobic ILs in IC. All of studied cations interact with the stationary phase surface by an ion-exchange mechanism, but each of them with different strength. Moreover, low correlation for polar IL cations is probably the consequence of their stronger (in comparison with AMIM, HMIM, and OMIM) ability to interact by hydrogen bonding or other hydrophilic interactions. For HMPy, EBzMIM, HMIM, and OMIM the correlation coefficients are approximately 0.98 (Table 4). The presence of long alkyl chains in the structure of the IL cations increases their hydrophobicity which probably determines their retention. It seems that the main interaction types are cation-exchange and hydrophobic, and that the participation of all polar bonds is slender.
Comparison of Calculated log P with log k w
log k w determined chromatographically and log P calculated by HyperChem software were both linearly regressed (Eq. 1). The coefficients characterizing this dependence are summarized in Table 5. The observed correlations were statistically significant, but very poor when log k w and log P values for all cations (i.e., alkylimidazolium, arylimidazolium, and pyridinium) were used for calculations (R 2 = 0.5911). A significant improvement occurred if only data for n-alkylimidazolium cations were subjected to linear regression (R 2 = 0.9689).
This effect is closely connected with the structure of the IL cations and, as a consequence, with interactions in which those salts can participate. MBPy, HMPy, BzMIM, and EBzMIM contain several double bonds (delocalized electrons), which can interact strongly through π–π interactions. The structures of the other IL cations differ only in the number and position of methyl groups on the imidazolium rings. Alkyl chains participate in hydrophobic interactions, therefore the presented correlation is high, because the log P values also indicate the ability of compound to interact through hydrophobic forces. The high R 2 values for IL with the same building unit are also connected with the nature of Eq. 1, which works well only for analytes with similar structure, not differing in functional groups. Chromatographic retention (expressed as log k w) and log P have different sensitivity to different interactions, especially hydrogen bonding.
Despite the low correlation for whole group of IL cations (Table 5), the column used in this study is suitable for prediction of log P for ILs with the same skeleton and alkyl substituents.
It does not seem to be very difficult to find a significant correlation for cations of a similar structure, where the differences in the retention are mainly because of one type of interaction. The major problem is to find such a good correlation for a mixture of substances of different structure, because competition among different types of interaction affects retention. However, more detailed study of a larger group of IL cations is needed. Such experiments are currently of interest to us.
Correlation of log k w Determined by IC with log P Determined by the Shake-Flask Method
Comparison of log k w data presented in this study with results obtained in several other investigations is complicated. First, there is little information about determination of log P for ILs by the shake-flask method, which is complicated for ionic compounds [29–31]. However, log P has been determined for [C4MIM][Cl] (−0.31), [C8MIM][Cl] (−0.27), [C10MIM][Cl] (−0.20), [C12MIM][Cl] (−0.14) [29], [C2MIM][PF6] (−1.82), [C4MIM][PF6] (−1.70) [30], [C4MIM][BF4] (0.003), [C4MIM][NO3] (0.0038), [C4MIM][PF6] (0.0220), [C4MIM][Cl] (0.004), [C4MIM][Br] (0.0033), [C2MIM][Tf2N] (0.09), [C4MIM][Tf2N] (0.11), [C6MIM][Tf2N] (1.42), and [C8MIM][Tf2N] (6.3) [31]. Only [BMIM][BF4] was also the subject of our study. Another difficulty in comparison those two hydrophobicity measures (log k w and log P) is concerned with differentiation of the IL cation and anion. In our investigation only cations were detected by the IC system, therefore log k w are characteristic for these species. Results obtained by use of the shake-flask method represent the hydrophobicity of the whole molecule, and, as was proved in earlier studies, the anion has a large effect on log P values [30, 31]. Although direct comparison of log P and log k w is not appropriate, some trends are obvious. Chromatographic hydrophobicity measured by use of IC is higher than that determined by use of the conventional method.
Retention Data for QSRR
In QSRRs studies, two kinds of input data are needed: chromatographic retention data for a number of analytes and analyte-related properties. One QSRR approach was described in an earlier paragraph: relating log k w to the logarithm of the n-octanol–water partition coefficient (log P). QSRR may be, however, performed in other ways, regressing log k w against structural descriptors obtained by calculation chemistry. Therefore, to achieve a QSRRs model, compounds must be represented by a molecular descriptor (Table 1).
Multiple linear regression was used to generate QSRR equations for the eleven ILs. Several structural descriptors were chosen for the studies, namely log P, A SAS, μ , PO, HE, and TE. Other descriptors were also considered and tested, but the equations derived using these were not statistically significant. Those descriptors were: net minimal charge (δ min), net maximum charge (δ max), binding energy, isolated atomic energy, electronic energy, heat of formation, molecular mass. Low correlation coefficients for log k w and δ min, δ max were especially unexpected as it was assumed that charge would have an important effect on retention. On the other hand such descriptors as PO, A SAS, and HE may be used for estimation of the ability of the analyte to undergo polar interactions.
Two-term QSRR equations using structural properties of IL cations obtained from molecular modeling calculations were found to describe isocratic retention in terms of log k w; these are presented in Table 6. The calculations were performed to determine the hydrophobicity of IL in a more complex manner. Use of the most statistically significant equations enable estimation of log k w, and, at the same time, log P (as it correlates well with log k w) (Table 5).
QSRR Analysis
The two most statistically significant equations with high coefficients of determination (R 2) used log P, μ, A SAS, and HE (Table 6). The first descriptor represents the ability of the IL cation to interact by hydrophobic forces. The dipole moment accounts for dipole–dipole and dipole-induced dipole attractive interactions of the analyte with the components of the competing mobile and stationary phase. A SAS and HE are connected with polar interactions. The first is the molecular surface area accessible to water. It represents hydrophilic interactions which occur between water and other molecules in such a way that the other molecules are attracted to water. HE is the energy released when IL cations undergo complete hydration in aqueous solution. The contributions of those four descriptors to log k w are dominant in the interaction of the IL cation and stationary phase molecules. Furthermore, R 2 for both equations is almost equal, which may suggest that those interactions, although varied in nature, have similar effects on log k w (Table 6).
However, the main purpose of this contribution is to determine which interaction is dominant and, consequently, to find the optimum properties for chromatographic determination of log k w. For this reason other statistically significant equations were calculated (Table 6).
A high coefficient of determination was also obtained when A SAS and the total energy of the IL cations were taken into consideration (R 2 = 0.9409) (Table 6). It seemed that both types of energy (TE and HE) affect the chromatographic retention of IL cations to a great extent, although the effect of TE is lower. On the other hand, when TE and HE are used in QSRR calculations together, they do not correlate well with log k w (R 2 = 0.8788).
Interesting are results presented for QSRR calculations with descriptors which refer to hydrophobic and polar interactions. The last equation in Table 6 combines A SAS and PO. PO gives information about molecular shape, the electronic charge distribution in the molecule. PO is the relative tendency of charge distribution by an external electric field, which may be caused by the presence of a nearby ion or dipole. The strength of the dispersive interactions of a substance containing no dipoles is related to its polarizability. The high R 2 value for this equation may suggest that for log k w of ILs hydrophilic and dispersive interactions are equally important. However, cation exchange is also important.
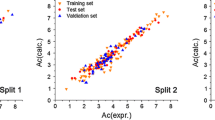
Use of QSRR for investigation of IL cations in IC enables evaluation of log k w by use of suitable descriptors. Figure 4 presents the correlation between experimentally determined log k w and the same property calculated by use of the equation with the highest correlation coefficient (R 2 = 0.9545).
Conclusion
The retention of IL cations strongly depends on the stationary phase surface and on mobile phase composition. If IL cations are eluted from a weak cation-exchange column with carboxylate and phosphonate cation-exchange sites and crown ether groups with acetonitrile–methanesulfonic acid as mobile phase, their retention mostly depends on their hydrophobic properties and they are eluted in reversed-phase order if the amount of ACN is <60% (v/v). However, the cations follow normal-phase elution when the amount of ACN is increased to 65% (v/v).
IC enables determination of chromatographic hydrophobicity log k w. Its values are higher than log P estimated by the shake-flask method, but they include many more interactions in which ILs participate during the partition process. The QSRR equations confirm the importance of hydrophobic interactions, dispersion interactions, and dipole–dipole and dipole-induced dipole attractive interactions in retention, as a result of their relationship with log k w.
This paper reports the first work describing use of data from ion chromatography of IL cations in QSRR. Further investigations should be undertaken, in particular to investigate the importance of stationary phase and mobile phase’s composition, and investigation of more ILs.
References
Huddleston JG, Visser AE, Reichert WM, Willauer HD, Broker GA, Rogers RD (2001) Green Chem 3:156–164S
Anderson JL, Armstrong DW, Wei GT (2006) Anal Chem 78:2892–2902
Pandey S (2006) Anal Chim Acta 556:38–45
Hough WL, Smiglak M, Rodríguez H, Swatloski RP, Spear SK, Daly DT, Pernak J, Grisel JE, Carliss RD, Soutullo MD, Davis JH, Rogers RD (2007) New J Chem 31:1429–1436
Koel M (2005) Crit Rev Anal Chem 35:177–192
Buszewski B, Studzińska S (2008) Chromatographia 68:1–10
Staff JR (1991) J Chromatogr A 547:484–487
Villagran C, Deetlefs M, Pitner WR, Hardacre C (2004) Anal Chem 76(7):2118–2123
Hao F, Haddad PR, Ruther T (2008) Chromatographia 67:495–498
Stepnowski P, Mrozik W (2005) J Sep Sci 28:149–154
Markowska A, Stepnowski P (2008) Anal Sci 24:1359–1361
Kaliszan R (1992) Anal Chem 64:619A–631A
Kaliszan R, van Straten MA, Markuszewski M, Cramers CA, Claessens HA (1999) J Chromatogr A 855:455–486
Kaliszan R (2007) Chem Rev 107:3212–3246
Kaar JL, Jesionowski AM, Berberich JA, Moulton R, Russell AJ (2003) J Am Chem Soc 125:4125–4131
Domańska U, Bogel-Lukasik E, Bogel-Lukasik R (2003) Chem Eur J 9:3033–3041
Ropel L, Belvéze LS, Aki SNVK, Stadtherr MA, Brennecke JF (2005) Green Chem 7:83–90
Lee SH, Lee SB (2009) J Chem Technol Biotechnol 84:202–207
Molíková M, Markuszewski MJ, Kaliszan R, Jandera P (2010) J Chromatogr A 1217:1305–1312
Dionex Corporation (2009) USA. http://www.dionex.com. Accessed 24 Sept 2009
Snyder R, Dolan JW (1998) Adv Chromatogr 38:115–185
Molíková M, Studzińska S, Kosobucki P, Jandera P, Buszewski B (2010) J Liq Chromatogr Relat Technol 33:225–238
Ruiz-Angel MJ, Berthod A (2006) J Chromatogr A 1113:101–108
Ruiz-Angel MJ, Berthod A (2008) J Chromatogr A 1189:476–482
Platts JA, Oldfield SP, Reif MM, Palmucci A, Gabano E, Osella D (2006) J Inorg Biochem 100:1199–1207
Stepnowski P, Storoniak P (2005) Environ Sci Pollut Res 4:199–204
Leo AJ (1993) Chem Rev 93:1281–1306
Ghose AK, Pritchett A, Crippen GM (1988) J Comput Chem 9:80–90
Domańska U, Bogel-Łukasik E, Bogel-Łukasik R (2003) Chem Eur J 9:3033
Chou C-H, Perng F-S, Wong DSH, Su WC (2003). In: Proceedings of the 15th symposium on thermophysical properties, Boulder, USA. http://www.symp15.nist.gov
Ropel L, Belveze LS, Aki SNVK, Stadtherr MA, Brennecke JF (2005) Green Chem 7:83–90
Acknowledgments
This research was supported by CEEPUS II scholarship numbers CII-PL-0004-04-0809-M-29668 and CII-PL-0004-04-0809-M-30343. The authors thank to Dr Michał Markuszewski (Faculty of Pharmacy, Chair and Department of Toxicology, Nicolaus Copernicus University) and Dr Detlef Jensen (Dionex) for scientific discussion. The authors are gratefully also to Foundation for Polish Science for scholarship in START programme.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Studzińska, S., Molíková, M., Kosobucki, P. et al. Study of the Interactions of Ionic Liquids in IC by QSRR. Chromatographia 73 (Suppl 1), 35–44 (2011). https://doi.org/10.1007/s10337-011-1960-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-011-1960-3