Abstract
Sex allocation has been a fertile topic in the development of evolutionary theory. The dominant models for vertebrates have provided predictions of sex ratios based on asymmetry in breeding success between sexes and the relative effect of local competition. In birds, empirical work has provided some support for these models, but has also generated apparently contradictory observations. Recent models have provided some predictions for both individual and population sex ratios, showing that the availability of high quality habitat patches and dispersal rates can critically affect both population- and individual-level expectations. We explore patterns in offspring sex ratio among chicks of the White Stork Ciconia ciconia, a large migratory bird species with bi-parental care, which does not show strong sexual dimorphism in size and survival patterns. We checked the hatching order and body weight of 342 nestlings from 124 broods, and their sexes were recorded over 4 years (2005–2008) in a dense healthy stork population in western Poland. Sex ratio in the study population was skewed to males (57.5%) which was consistent between years. We have assumed that chick age was directly related to their size. The heavier (and assumed older) chicks were significantly dominated by males.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The variation in the primary sex ratio in vertebrates is poorly understood compared with invertebrates, probably because of the relative complexity of sex allocation and life history in vertebrates (Badyaev et al. 2002; Rutkowska and Badyaev 2008). Although there is a growing literature on facultative sex ratio adjustment in chromosomal sex-determining vertebrate taxa (birds, mammals), the consistency of results is often low between studies and species (e.g. Donald 2007). Griffin et al. (2005) argued that across species the selection on sex ratio adjustment may be variable due to differences in breeding system, sexual dimorphism, and life-histories, whereas within species such variability in selection is less straightforward. Studies investigating the repeatability of facultative sex ratio adjustment in the same species between years, and for the same individuals, are scarce, yet crucial to determine with confidence the frequency of sex ratio modification in specific taxa. To date, within-species studies have mainly used large diurnal raptors and owls as the models (Daan et al. 1996; Blanco et al. 2003a, b; Väli 2004). This is because such species have a strong tradition of long-term studies (Väli 2004; Kekkonen et al. 2008), and are large, so that genetic material (usually blood) is easy to collect. Last but not least, many raptors have a large dimorphism (regular or reversed) between the sizes of the sexes, which produces clear patterns to researchers identifying which sexes are worth more investment by avian parents (Blanco et al. 2003a; Hipkiss and Hörnfeldt 2004; Kekkonen et al. 2008). However, other large birds also have similar food composition and similar population response to major food sources, i.e. to voles Microtus spp., for example the White Stork Ciconia ciconia (Creutz 1985; Schulz 1998; Tryjanowski and Kuźniak 2002). Therefore, in a similar way, the sex ratio among nestlings in these other species could be manipulated by parents according to food availability or generally via a seasonal (year) effect (including weather conditions; Tryjanowski et al. 2004), which influences the reproduction of these species (Väli 2004; Kekkonen et al. 2008). In contrast to many birds of prey and to owls, the White Stork does not have a very obvious sexual dimorphism, either in size (males are slightly bigger and heavier, up to 5%) or in energy requirements (Schulz 1998; Baos et al. 2006; Cwiertnia et al. 2006; Kwiecinski and Tryjanowski 2009). Even studies in survival patterns do not show sex differences (Kanyamibwa et al. 1990; Schaub et al. 2005), and a study of nestlings did not show sexual differences in hatching order or sibling position of chicks in the nest (Aguirre and Vergara 2007). Therefore, it is not easy to predict which chick sex may be favoured by parents under different conditions. However, a study of a related species, the Black Stork C. nigra, showed a female-skewed sex ratio in a Portuguese population (Fernandes et al. 2006).
Obviously, the population sex ratio is a product of sex ratio among broods of breeding pairs and the mechanism of realised sex ratio started at the individual bird level. Most species of birds, including White Stork, lay one egg per day until a clutch is complete, and the eggs hatch in approximately the order in which they are laid (however, with exceptions; see for example Blanco et al. 2003b; Cook and Monaghan 2004). Parents may modify the often strong effects of hatching asynchrony on survival of male and female offspring either by providing different resources to offspring of different laying order (Badyaev et al. 2002; Blanco et al. 2003a; Szekely et al. 2004) or by changing the sex ratio of offspring in relation to their position in the clutch (Badyaev et al. 2002; Blanco et al. 2003a). Thus, given the ubiquitous effects of hatching order on growth and survival of offspring and environmental variation in the costs and benefits of raising sons and daughters (Badyaev et al. 2002; Szekely et al. 2004), selection may favour the evolution of a mechanism that enables breeding females to adjust the sex and growth of offspring simultaneously in relation to their position in the laying order (Badyaev et al. 2002; Blanco et al. 2003a).
In this study, we check the classical question of the sex ratio among chicks in a population of White Storks. Secondly, we check if the sex ratio differed between years and broods of different size. Thirdly, we check how hatching order was related to the sex of chicks. Finally, we discuss, for the studied population, why there existed a male-skewed sex ratio and what the potential consequences for the population might be (Quader 2005). This is also urgent information, because the White Stork is of high conservation interest, and information on population sex ratio plays an important role in understanding the population dynamics of this particular species (Olsson 2006).
Methods
Studies were performed in 2005–2008 in western Poland in an area between 51°40′–54°38′N and 14°42′–17°33′E, in habitats with a relatively high density of the White Stork (for more details, see Tryjanowski et al. 2005; Kulczykowska et al. 2007). All these White Storks bred in open nests on roofs, in the tops of trees, or on electricity posts. Chicks hatch after approximately 1 month of incubation, but rely on both parents for food and protection during the next 70–80 days. Our studies were carried out during their nesting season, and all samples were collected between 23 June and 4 July in each year.
Blood samples (5 ml each) were collected from nestlings via a veni-puncture of the brachial vein. Each chick was retrieved from the nest and placed in individual ventilated cotton sacks. White Stork chicks displayed very little response to handling. Body mass (to the nearest 50 g) was determined using a Pesola spring balance. Age (and thus also hatch date) was estimated by measurement of the bill length (with an accuracy of 1 day) according to Kania (1988). This is generally considered to be a reliable ageing method, and the gaps (2–3 days) between successive hatchings were still very apparent at the time of our recording. Additionally, age of chicks was estimated from body mass using the formula of Tortosa and Castro (2003), and both estimates of age were highly significantly positively correlated (r = 0.855, P < 0.001). The mean (±SE) age of chicks during sampling was 37.1 ± 0.48 days.
As White Storks do not exhibit strong sexual dimorphism (Schulz 1998; Cwiertnia et al. 2006), we resorted to molecular sexing of the birds by using the cellular fraction of the blood as a source of DNA. Whole genomic DNA was extracted using DNA easy Blood & Tissue Kit (Qiagen, Hilden, Germany) following the manufacturer’s standard protocol. CHD-W and CHD-Z gene fragments were PCR amplified with primers 2550F and 2718R (Fridolfsson and Ellegren 1999). PCRs were carried out in a final volume of 25 μl containing 1× reaction buffer (Fermentas, Vilnius, Lithuania), 1.5 mM MgCl2, 0.1 mM dNTPs, 0.25 μM each primer, 1.25 U Taq polymerase (HiFi; Novazym, Poznan, Poland), and 100 ng of DNA template using a thermocycling profile of one cycle of 3 min at 96°C followed by 35 steps of 10 s at 95°C, 15 s at 50°C, 1 min at 72°C, with a final step of 5 min at 72°C. Amplification products were separated by electrophoresis on 1.5% agarose gel. It was not possible to determine the sex of five chicks whose blood samples were damaged, so gender information on these birds is missing.
It is worth noting that most adults (ca. 90%) in the studied population were not ringed. Therefore, it follows that we do not have exact information on how many females/pairs were represented in the sample in more than 1 year. Consequently, results from statistical analysis may need to be treated with caution because the exact number of independent pairs (replicates) is unknown. However, the same problem occurs in other studies on long-lived birds when adults are not easy to catch (e.g. Väli 2004; Fernandes et al. 2006; Kekkonen et al. 2008).
Statistical analyses were conducted using the Minitab v.15 package. For most analyses, single summary values per brood were used. The sole exception was when comparing male and female weights when individual weights of chicks were subjected to a nested ANOVA with chick sex nested within brood.
Results
In 2005–2008, a total of 342 nestlings from 124 broods were sexed. The mean ± SE brood size was 2.44 ± 0.17 (n = 18) in 2005, 2.56 ± 0.15 (n = 34) in 2006, 3.11 ± 0.15 (n = 36) in 2007 and 2.89 ± 0.13 (n = 36) in 2008. The difference in brood size between years was statistically significant (ANOVA, F 3,120 = 3.73, P = 0.013).
Among sexed chicks, 150 (43.9%) were female and 192 (56.1%) were male. A one-sample t test on the proportion of males in the 124 broods showed a significant difference from parity (mean 57.5% male, t = 2.72, P = 0.007). No significant differences were found between years in the sex ratio (computed from the percentage of male chicks in each brood, ANOVA, F 3,120 = 0.33, P = 0.80). Males were more common than females in all brood size categories, except those with four chicks (Table 1).
Males dominated as first hatched chicks across the study (Table 2) except in 2005 where only marginal significance was achieved with a small sample size (n = 18 broods). Overall, the oldest chick in 80% of the broods was male. This appeared consistent irrespective of brood size (Table 1).
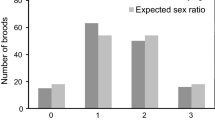
Male dominance was greatest among the first hatched, and reversed lower in the hatching order (Fig. 1).
Analysis of body weight (two-way ANOVA: brood i.d. and sex) suggested that male chicks were significantly heavier (by about 10%) than female chicks (F 1,217 = 63.14, P < 0.001, males 2,918 ± 22 vs females 2,634 ± 26). However, this was to be expected given that male chicks were typically older. Adding age as a covariate into the model still suggested heavier male chicks but the differential was reduced to ca. 3% (F 1,216 = 6.05, P = 0.015, males 2,871 ± 16 g vs females 2,800 ± 21 g).
Discussion
Throughout this study, we have assumed that the size of chicks reflected their age and that relative size reflected hatching order. If, however, some chicks were preferentially fed, or preferentially acquired food, the possibility exists that they may have grown faster than their siblings. However, without a very invasive nest study, which would be logistically difficult and would likely adversely disturb the nursing parents, this can only be conjecture. There is no doubt that sex ratios are male-skewed. Furthermore, we believe that age estimation from chick bill length is an accurate method and that size reflects age, and we draw our conclusions based on this assumption.
Our study shows that the secondary sex ratio (sex ratio at hatching and nestling phases) in the White Stork chicks was male-biased. The same is true for the tertiary sex ratio (at fledging), since all chicks from which blood was collected survived to fledging the nest. In other studies on White Stork, the number of nestlings that survived the first 2 weeks was also quite similar to the final number of fledglings because predation and starvation were very uncommon (Andrzejewska et al. 2004; Aguirre and Vergara 2007), and nestlings are only vulnerable to inclement weather during the first 20 days of life (Jovani and Tella 2004; Profus et al. 2004). On the other hand, it is known that the White Stork may reduce brood size. Tortosa and Redondo (1992) observed that stork parents may kill their smaller chick, which, according to our findings, might be female. Sasvari et al. (1999) also noted that cases of brood reduction were related to testosterone concentrations in chick blood. The first-hatched chicks, which had high plasma testosterone levels (hence mainly males although chicks were not sexed in the original study), responded faster to the feeding parent and received more food than did their younger siblings.
However, during a separate detailed monitoring of 25 nests in our study area, we found only one egg and one chick rejected from nests (M. Bochenski, unpublished observations). Therefore, we assume that the primary sex ratio (egg phase) would also be similar to our other findings (male-biased). If parents actively reduce the number of females during the nestling stage, we would expect this to occur during years with poor weather and reduced food sources. However, we found, in the studied population, that the male-biased sex ratio was similar between years, despite the large differences in mean brood size likely due to food availability. Our study only covered 4 years but, unlike other studies (e.g. Jovani and Tella 2004; Tryjanowski et al. 2004, 2005), we did not detect a year effect on the sex ratio among chicks. However, in the White Stork, a strong year effect on population productivity is well known. Very good or very poor years may occur only once per decade, therefore confirmation of our findings, or otherwise, in long-term studies should be encouraged.
Older chicks were usually male, which supports the hypothesis to produce older offspring as the more valuable sex (Badyaev et al. 2002; Blanco et al. 2003b; Kekkonen et al. 2008). Interestingly, the situation was very similar in broods of different sizes, meaning that even storks in relatively poorer condition and/or which bred in poorer habitats (i.e. those with smaller brood sizes) had a tendency to produce the first offspring as male. The hatching order sex bias suggests that males would benefit from earlier hatching, and consequently, as bigger chicks, may survive better. Therefore, a simple suggestion may be that the first chicks originate from extra-pair copulations (Nager et al. 1999; Quader 2005). However, we may exclude this suggestion, because a detailed study by Tortosa and Redondo (1992) showed that in White Stork (even in colonial conditions) extra pair copulations were very low (below 1%).
If male chicks are produced more often, tend to be older and therefore survive better (at least in the nest stage), the result will be a male skew ratio in the local population. However, we do not know what the sex ratio among adult storks is in the study area. Moreover, to the best of our knowledge, there are no population data on the sex ratio of breeding White Storks, but there are some suggestions of more male than female adults (Creutz 1985; Chernetsov et al. 2006; Olsson 2006). Could males reach sexual maturity before females and thus start to migrate from Africa to breeding areas at a earlier age, and pay the price in lower survival? As a consequence, females, as the sex in lower numbers, may be more successful in finding partners of greater fitness with which to breed. This may explain the situation in a Spanish White Stork population, where younger, weaker chicks become the best breeders in the future (Aguirre and Vergara 2007). However, those authors did not find a sex bias in hatching order or body mass. Paradoxically, better nest conditions could exert a negative effect on later survival, and it may not always be older chicks, especially males, that have better prospects in their future. However, this is very speculative, but is worthy of closer scrutiny.
Taking account of the suggestions that the sex ratio may differ between populations of the same species (Fernandes et al. 2006; Kekkonen et al. 2008), it is worth noting that our White Stork data were collected in a dense healthy population of the species, mainly located in the Odra river valley (Tryjanowski et al. 2005). We may predict that, in more intensively used farmland, where conditions for the White Stork are worse and where density and breeding success are lower (Tryjanowski and Kuźniak 2002; Olsson 2006; Sæther et al. 2006), the sex ratio may be more skewed to females. However, to our knowledge, data on sex ratios of White Storks in poor habitats do not yet exist.
Zusammenfassung
Schlüpfen beim Weißstorch Ciconia ciconia die Männchen zuerst und dominieren sie das Geschlechterverhältnis bei den Küken?
Die Geschlechterverteilung ist ein bedeutender Gegenstand in der Entwicklung von Evolutionstheorien. Die vorherrschenden Modelle für Wirbeltiere geben Vorhersagen zu Geschlechterverhältnissen, basierend auf der Asymmetrie im Bruterfolg zwischen den Geschlechtern sowie relativen Effekte auf die lokale Konkurrenz. Empirische Untersuchungen an Vögeln haben diese Modelle bestätigt, gleichzeitig jedoch auch offensichtlich widersprüchliche Beobachtungen erbracht. Aktuelle Modelle ergaben Vorhersagen sowohl für individuelle als auch auf Populationsebene bezogene Geschlechterverhältnisse. Diese zeigen, dass die Verfügbarkeit hochwertiger Habitate sowie Dispersionsraten die Erwartungen sowohl auf Populations- als auch auf Individuenbasis entscheidend beeinflussen können. Wir untersuchten Muster im Geschlechterverhältnis von Weißstorchküken Ciconia ciconia. Diese große Zugvogelart zeigt keinen starken Sexualdimorphismus in Größe und Überlebensraten. Darüber hinaus beteiligen sich beide Partner an der Brutpflege. Wir erfassten Schlupfreihenfolge und Körpermasse von 342 Küken aus 124 Bruten und ihr Geschlecht über vier Jahre (2005–2008) in einer dicht besiedelten „gesunden” Weißstorchpopulation in Westpolen. Das Geschlechterverhältnis in der beobachteten Population war in allen Jahren hin zu den Männchen (57.5%) verschoben, Zudem nahmen wir an, dass das Kükenalter direkt mit deren Größe zusammenhängt. Die schwereren (und angenommen älteren) Küken waren signifikant häufiger Männchen.
References
Aguirre JI, Vergara P (2007) Younger, weaker white stork (Ciconia ciconia) nestlings become the best breeders. Evol Ecol Res 9:355–364
Andrzejewska I, Tryjanowski P, Zduniak P, Dolata PT, Ptaszyk J, Cwiertnia P (2004) Toxoplasma gondii antibodies in the white stork Ciconia ciconia. Berl Munch Tierarztl Wschr 117:274–275
Badyaev AV, Hill GE, Beck M, Dervan AA, Duckworth RA, McGraw KJ, Nolan PM, Whittingham LA (2002) Sex-biased hatching order and adaptive population divergence in a passerine bird. Science 295:316–318
Baos R, Blas J, Bortolotti GR, Marchant TA, Hiraldo F (2006) Adrenocortical response to stress and thyroid hormone status in free-living nestling white storks (Ciconia ciconia) exposed to heavy metal and arsenic contamination. Environ Health Perspect 114:497–501
Blanco G, Martinez-Padilla J, Davila JA, Serrano D, Vinuela J (2003a) First evidence of sex differences in the duration of avian embryonic period: consequences for sibling competition in sexually dimorphic birds. Behav Ecol 14:702–706
Blanco G, Martínez-Padilla J, Serrano D, Dávila JA, Viñuela J (2003b) Mass provisioning to different-sex eggs within the laying sequence: consequences for adjustment of reproductive effort in a sexually dimorphic bird. J Anim Ecol 72:831–838
Chernetsov N, Chromik W, Dolata PT, Profus P, Tryjanowski P (2006) Sex-related natal dispersal of white storks (Ciconia ciconia) in Poland: how far and where to? Auk 123:1103–1109
Cook MI, Monaghan P (2004) Sex differences in embryo development periods and effects on avian hatching patterns. Behav Ecol 15:205–209
Creutz G (1985) Der Weißstorch Ciconia ciconia. Ziemseng, Wittenberg Lutherstadt
Cwiertnia P, Kwiecinski Z, Kwiecinska H, Wysocki A, Tryjanowski P, Ollson O (2006) Sexing of white stork Ciconia ciconia based on biometric characters. In: Tryjanowski P, Sparks TH, Jerzak L (eds) The white stork in Poland: studies in biology, ecology and conservation. Bogucki, Poznań, pp 423–429
Daan S, Dijkstra C, Weissing FJ (1996) An evolutionary explanation for seasonal trends in avian sex ratios. Behav Ecol 7:426–430
Donald PF (2007) Adult sex ratios in wild bird populations. Ibis 149:671–692
Fernandes M, Borges C, Simoes F, Caballero JM, Pacheco C, Franco C (2006) Molecular sexing of the black stork Ciconia nigra: sex ratios in the Portuguese population. Biota 7:31–36
Fridolfsson AK, Ellegren H (1999) A simple and universal method for molecular sexing of non-ratite birds. J Avian Biol 30:116–121
Griffin AS, Sheldon BC, West SA (2005) Cooperative breeders adjust offspring sex ratios to produce helpful helpers. Am Nat 166:628–632
Hipkiss T, Hörnfeldt B (2004) High interannual variation in the hatching sex ratio of Tengmam’s owl broods during a vole cycle. Popul Ecol 46:263–268
Jovani R, Tella JL (2004) Age-related environmental sensitivity and weather mediated nestling mortality in white storks Ciconia ciconia. Ecography 27:611–618
Kania W (1988) Investigations of white stork (Ciconia ciconia) hatching phenology based on bill measurements of nestlings. Ring 134–135:13–19
Kanyamibwa S, Schierer A, Pradel R, Lebreton J-D (1990) Changes in adult annual survival rates in a western European population of the white stork Ciconia ciconia. Ibis 132:27–35
Kekkonen J, Kolunen H, Pietiäinen H, Karell P, Brommer JE (2008) Tawny owl reproduction and offspring sex ratios under variable food conditions. J Ornithol 149:59–66
Kulczykowska E, Kasprzak M, Kalamarz H, Kuriata M, Nietrzeba M, Jerzak L, Kaminski P (2007) Melatonin and thyroxine response to pollution in white stork nestlings (Ciconia ciconia): aspects of rhythmicity and age. Comp Biochem Physiol 146C:392–397
Kwiecinski Z, Tryjanowski P (2009) Sex differences in digestive efficiency of the white stork Ciconia ciconia under experimental conditions. Folia biol (Krakow) 57:193–198
Nager RG, Monaghan P, Griffiths R, Houston DC, Dawson R (1999) Experimental demonstration that offspring sex ratio varies with maternal condition. Proc Natl Acad Sci USA 96:570–573
Olsson O (2006) Genetic origin and success of reintroduced white storks. Conserv Biol 21:1196–1206
Profus P, Tryjanowski P, Tworek S, Zduniak P (2004) Intrapopulation variation of egg size in the white stork (Ciconia ciconia) in southern Poland. Pol J Ecol 52:75–78
Quader S (2005) Mate choice and its implications for conservation and management. Curr Sci 89:1220–1229
Rutkowska J, Badyaev AV (2008) Meiotic drive and sex determination: molecular and cytological mechanisms of sex ratio adjustment in birds. Phil Trans Roy Soc Lond B 363:1675–1686
Sæther B-E, Grøtan V, Tryjanowski P, Barbraud C, Engen S, Fulin M (2006) Climate and spatio-temporal variation in the population dynamics of a long distance migrant, the white stork. J Anim Ecol 75:80–90
Sasvari L, Hegyi Z, Peczely P (1999) Brood reduction in white storks mediated through asymmetries in plasma testosterone concentrations in chicks. Ethology 105:569–582
Schaub M, Kania W, Köppen U (2005) Variation of primary production during winter induces synchrony in survival rates in migratory white storks Ciconia ciconia. J Anim Ecol 74:656–666
Schulz H (1998) Ciconia ciconia white stork. BWP Update 2:69–105
Szekely T, Cuthill IC, Yezerinac S, Griffiths R, Kis J (2004) Brood sex ratio in the Kentish plover. Behav Ecol 15:58–62
Tortosa FS, Castro F (2003) Development of thermoregulatory ability during ontogeny in the white stork Ciconia ciconia. Ardeola 50:39–45
Tortosa FS, Redondo T (1992) Motives for parental infanticide in white storks Ciconia ciconia. Ornis Scand 23:185–189
Tryjanowski P, Kuźniak S (2002) Size and productivity of the white stork Ciconia ciconia population in relation to common vole Microtus arvalis density. Ardea 90:213–217
Tryjanowski P, Sparks TH, Ptaszyk J, Kosicki J (2004) Do white storks Ciconia ciconia always profit from an early return to their breeding grounds? Bird Study 51:222–227
Tryjanowski P, Jerzak L, Radkiewicz J (2005) Effect of water level and livestock on the productivity and numbers of breeding white storks. Waterbirds 28:378–382
Väli U (2004) Sex ratio of lesser spotted eagle Aquila pomarina nestlings in good and poor breeding years. Bird Study 51:189–191
Acknowledgments
We thank all farmers and students who participated in this study, and Joanna Rutkowska, Jakub Z. Kosicki, Thomas W.P. Friedl and an anonymous referee for comments on an earlier version of the manuscript. T.H.S. was formerly employed at CEH Monks Wood. The study was undertaken following the Guidelines of the European Union Council and the current laws in Poland, according to the Ethical Commission (permission number: 05/2005).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Friedl.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Tryjanowski, P., Sparks, T.H., Bochenski, M. et al. Do males hatch first and dominate sex ratios in White Stork Ciconia ciconia chicks?. J Ornithol 152, 213–218 (2011). https://doi.org/10.1007/s10336-010-0571-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-010-0571-3