Abstract
Stable carbon- (δ13C), nitrogen- (δ15N) and hydrogen (δD) isotope profiles in feathers of migratory Great Reed Warblers Acrocephalus arundinaceus recaptured for 2 or more years in 6 successive years were examined to test whether the isotope profiles of individual warblers appeared to be consistent between years. Similar isotopic signatures in successive years suggested that individual birds tended to return and grow their feathers in Afro-tropical wintering habitats that generate similar δ13C, δ15N and δD signatures. Previous studies have shown that Great Reed Warblers exhibit strong natal and breeding philopatry, with most of the surviving birds returning to the breeding site. The present study of feather δ13C, δ15N and δD isotopic values demonstrate the year-to-year fidelity might also include the African moulting sites in this migratory species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many migratory bird species are known to exhibit strong philopatry to their breeding and wintering grounds (e.g. Nisbet and Medway 1972; Baker 1978; Curry-Lindhal 1981; Bibby and Green 1981; Greenwood and Harvey 1982; King and Hutchinson 2001; Holmes and Sherry 1992). The evolutionary advantage of being faithful to breeding and/or non-breeding grounds includes the use of acquired experience and prior knowledge to rely on potentially good breeding or wintering habitat, nesting or roosting territory, foraging sites, best mate choice and predatory avoidance (Greenwood and Harvey 1982).
Although numerical data confirming these are rather scarce, it has been shown that some Acrocephalus species exhibit strong site fidelity and return to same area in Afro-tropical wintering grounds both between and within winters (e.g. Ash 1981; Hanmer 1986; King and Hutchinson 2001). A total of about 60 migrant species are reported to return to a general area in Africa in subsequent winters (Salewski et al. 2000). Earlier, using winter grown feather stable-isotope profiles, we showed site fidelity and habitat in nine migratory species moving through sub-Saharan Africa (Yohannes et al. 2007). However, no study (at least to our current knowledge) has confirmed habitat choice (trophic-level fidelity) and philopatry in individually marked migratory birds at Afro-tropical moulting grounds and northern latitude breeding sites between and within years using stable-isotope techniques.
To date, studies on site fidelity have largely depended on scattered observations and ringing recoveries. The low probability of ringing recoveries between regions visited by Palearctic-African migratory birds, challenge the functional use of the method on migration studies. Hence, several other approaches, such as measurement of tissue stable-isotope values (reviewed by Hobson 2005), are being applied in animal ecology studies. The technique is based on the application of natural abundance and composition of stable-carbon, nitrogen and hydrogen isotope values in food webs and avian tissues to provide information on habitat and trophic level use, diet and geographical origin (e.g. Lajtha and Michener 1994; Hobson and Wassenar 1997; Kelly 2000).
We applied both stable-isotope and ringing recoveries approach on a breeding population of the Great Reed Warbler Acrocephalus arundinaceus in a study site in Sweden to study the extent of moulting area philopatry during non-breeding season. The Great Reed Warbler is a small long-distance migratory passerine that breeds in reed-filled water areas throughout the Palearctic (Cramp 1992). Ringing recoveries of birds ringed in our population indicate that our study population migrates to tropical West Africa; one ringing recovery is from the Ivory Cost in November and one from southern Tchad in December (Bensch 1993). However, whether this population is also crossing the equator in mid-winter to reach areas in Congo is possible but not known (De Roo and Deheegher 1969; Hedenström et al. 1993).
Using individually marked birds at a breeding ground, our main objective was to assess whether there exists habitat philopatry in the non-breeding season by comparing stable-isotope profiles in feather (grown in the non-breeding season) samples collected from birds that are captured and recaptured in six successive breeding years. In our study species, the timing of autumn migration towards the south differs between the age classes. Hence, adult birds might start migration, reach their wintering ground and commence moulting earlier than juveniles. The moult-schedule difference might affect the stable-isotope signatures. Age effect was tested using birds that were caught as yearlings and re-caught as an adult in subsequent year(s).
Methods
Sample collection
Between spring 1999 and 2004, we collected the second outermost tail feathers from most Great Reed Warblers breeding at Lake Kvismaren (59°10′N 15°25′E), located in south central Sweden. Details about the study population and the field methods can be found in Bensch et al. (1998). Within the 6 consecutive study years, a total of 65 feather samples were collected and analysed from 29 individual birds that were re-caught for at least ≥2 years.
Briefly, in spring 2000, we re-captured seven individuals which were first caught in 1999 and feathers samples were taken; and two other individual birds were re-caught in 2001 and further feather samples were collected. In 2001, we also re-caught and collected feather samples from one other bird that was first caught in 2000. In 2002, we re-captured a total of ten birds, which were first caught in 1999 (n = 4), 2000 (n = 2) and 2001 (n = 4). One of these individual birds was repeatedly caught three times: first in 1999 and then in 2001 and 2002. In spring 2003, we caught seven individuals that were first caught in previous years (among which one bird was also caught in 1999) and one other bird which was first caught in 1999 and another in 2000. In 2004, we caught three birds from the year 2003 and one bird from 2002. A single individual bird was controlled and feather samples were collected for the three consecutive years between 1999 and 2001. Two other birds were also monitored for three consecutive years between 2002 and 2004.
Stable-isotope measurements
Carbon and nitrogen
For the δ13C and δ15N measurements, cleaned sub-samples of approximately 0.5 mg of feather were weighed into small tin cups and combusted in a Eurovector (Milan, Italy) elemental analyzer. The resulting N2 and CO2 gases were separated by gas chromatography and admitted into the inlet of a Micromass Isoprime isotope ratio mass spectrometer (Manchester, UK) for determination of 15N/14N and 13C/12C ratios. Measurements are reported in δ-notation relative to the PDB for carbon and atmospheric N2 standard in parts per thousand deviations (‰). Typical precision of analyses was ± 0.5‰ for δ15N and ± 0.2‰ for δ 13C. The standard for δ15N is atmospheric nitrogen and Peedee belemnite for δ13C. Egg albumin was used as daily reference material.
Deuterium
Deuterium measurements on feathers and standards were performed on H2 derived from high-temperature flash pyrolysis of feathers and Elemental Analyser-Isotope Ratio Mass Spectrometry calibrated against standard reference material and quality control checks. Briefly, cleaned and dried feather samples were filled into silver capsules which were left open for a period of not less than 4 days to allow the exchangeable hydrogen in the sample chitin to fully equilibrate with the moisture in the laboratory air. In addition, we analysed multiple samples of BWB-II (whale baleen), with a known non-exchangeable δ2HV-SMOW value, and our eggshell membrane standard, independently measured by Len Wassenaar, NWRI, Saskatoon, Canada. Multiple keratin replicate standards, whose non-exchangeable δD values are known, were used for correcting uncontrolled isotopic exchange between samples and ambient water vapour (Wassenaar and Hobson 2000). Thus, values reported here are equivalent to non-exchangeable feather hydrogen (Wassenaar and Hobson 2003). Deuterium analyses of feathers were undertaken at the Iso-analytical laboratory, England.
Data analysis
Within-individual repeatability analyses were calculated according to Lessells and Boag (1987). Paired t-test was used to assess age-effect (first calendar year vs adults) on feather stable isotope signatures.
Results
Feather stable isotopes
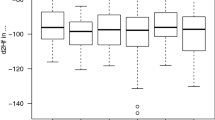
There was a significant repeatability (Fig. 1) in feather δ13C (R = 0.61, F 28,36 = 4.52, P < 0.001) and δ15N (R = 0.33, F 28,36 = 2.08, P = 0.02), and δD values (R = 0.28, F 28,36 = 1.88, P = 0.04). There was no significant age-related effect on the three elements analysed (paired t-test, δ13C: t 9 = 0.72, P = 0.49; δ15N: t 9 = 0.23, P = 0.79; δD: t 9 = 0.25, P = 0.81).
Winter grown feather δ13C, δ15N and δD values in 29 individual Great Reed Warblers Acrocephalus arundinaceus. Within the 6 consecutive study years, a total of 65 feather samples were collected (1999 = 12, 2000 = 9, 2001 = 9, 2002 = 16, 2003 = 13 and 2004 = 6). Solid and dashed lines connect individual data points with consecutive and non-consecutive years, respectively
Discussion
The main finding of this analysis is that the 65 feather samples collected from 29 individuals in 6 different moulting years showed significant repeatability for feather δ13C, δ15N and δD values. The strong between-year repeatability in feather isotope values of the Great Reed Warbler generally indicates that individual birds moult in similar habitats and depend on similar diets through the moulting period in different years. Although the observed isotopic homogeneity for feathers from the same individual does not guarantee that birds return to the exactly the same geographic spot in successive years, it clearly indicates that they selected habitats that generated similar isotope values within the potential moulting range.
We found no difference between yearlings and older birds in feather isotopic composition. Although we have a restricted data from first calendar year birds (n = 9), the mean δ13C, δ15N and δD feather values were relatively consistent among age-groups. In addition to the spatial similarity of the moulting ground, these results imply that age-related moult schedule did not affect the isotope signatures, at least for the nine individuals monitored here.
Ecosystems with C3 and C4 plant photosynthetic pathways produce different δ13C signatures (Smith and Epstein 1971; Koch et al. 1995). Feather δ13C values of Great Reed Warbler reflect utilization of C4 biome during moult. Stable isotope analyses of feathers from three adult Great Reed Warblers collected at breeding grounds in Portugal (Neto et al. 2006) showed a higher δ13C values (ca. + 6‰), but a similar δ15N values (mean ± SE: 10.3 ± 0.74) than the birds in our study. Though the Portuguese sample size is small, it suggests that these birds moult in an even more C4 extreme dependent biome than the birds from Sweden.
Consumer tissue stable-nitrogen isotopes (δ15N) are mainly used to infer food web trophic levels (e.g. Minagawa and Wada 1984; Kelly 2000). The significant repeatability δ15N between years and age groups would suggest a similar diet between winter moult seasons (Hobson 1999; Kelly 2000).
In North America, δD values of feathers closely reflectthat of the growing season average precipitation of the locations where the feathers were grown (Chamberlain et al. 2000; Hobson and Wassenaar 1997; but see also Langin et al. 2007). Based on this, and our results that showed a significant repeatability in feather δD, the birds are predicted to have returned to the same geographic location for moulting.
As shown by Hansson et al. (2002), the species is characterized by a high level of philopatry to natal and breeding sites. In a previous study comparing philopatric and immigrant Great Reed Warblers in Sweden, we have observed that the lifetime fitness of philopatric birds was higher than for immigrants (Bensch et al. 1998). Philopatry is generally believed to help maintain the adaptation of a population to a very specific environment (e.g. Clobert et al. 1988; Pärt 1994). Provided that one advantage of philopatry is based on acquired experience for better feeding sites and avoidance of predators, Great Reed Warblers that depend on similar winter habitat may benefit from that. However, our results do not exclude the alternatively hypothesis that high philopatry to wintering habitat could partly be due to the localised and uneven distribution of the preferred wintering habitats in Africa. This passerine bird mainly depends on large reed beds, often with some bushes around. These specific and preferred habitats are limited in their occurrence and distribution in Africa; the birds might show obligatory philopatry and a shift in habitat as a prevailing strategy to avoid unsuitable habitats.
Conclusion
The stable δ13C, δ15N and δD isotope profiles in feathers of 29 Great Reed Warblers that were monitored for ≥2 years in 6 study years were consistent in their repeatability between years. In general, our results show some degree of year-to-year philopatry in selecting a moulting habitat that generated a similar δ13C, δ15N and δD values outside the breeding season. Our use of stable isotope measurements on multiple elements, coupled with earlier studies using ringing recoveries and capture–recapture studies (e.g. Hansson et al 2002), provides a strong basis to generally conclude that the species shows a strong tendency to a year-round philopatry in both breeding and non-breeding seasons. Although with a smaller sample size, in a study of Willow Warbler Phylloscopus trochilus feathers presumably moulted in Africa and collected in Sweden in successive years (Bensch et al. 2006), there were high and significant repeatabilities for both δ13C (0.71) and δ15N (0.77).
Zusammenfassung
Untersuchung stabiler Isotope deutet auf Ortstreue von Wintermausergebieten beim Drosselrohrsänger Acrocephalus arundinaceus
Mit der Methode stabiler Isotope in Federn wurde bei Drosselrohrsängern, die innerhalb von sechs Jahren zweimal oder mehrfach kontrolliert wurden, untersucht, ob die Isotopenprofile der einzelnen Individuen konstant waren. Die Ähnlichkeit der Isotopensignaturen einzelner Individuen in folgenden Jahren legt nahe, dass sie regelmäßig zur Gefiedermauser zu den gleichen Winterbiotopen mit ähnlichen δ13C, δ15N und δD zurückkehren. Bisherige Studien am Drosselrohrsängern haben ausgeprägte Geburts- und Brutortstreue der zurückkehrenden Vögel gezeigt. Diese Studie unterstreicht die mögliche Bedeutung bestimmter afrikanischer Mausergebiet für diese Zugvogelart.
References
Ash JS (1981) Bird-ringing results and ringed bird recoveries in Ethiopia. Scopus 5:85101
Baker RR (1978) The evolutionary ecology of animal migration. Hodder & Stoughton, London
Bensch S (1993) Costs, benefits and strategies for females in a polygynous mating system: a study on the great reed warbler. Doctoral Dissertation. Lund University
Bensch S, Bengtsson G, Åkesson S (2006) Patterns of stable isotope signatures in willow warbler Phylloscopus trochilus feathers collected in Africa. J Avian Biol 37:323–330
Bensch S, Hasselquist D, Nielsen B, Hansson B (1998) Higher fitness for philopatric than for immigrant males in a semi-isolated population of great reed warblers. Evolution 52:877–883
Bibby CJ, Green RE (1981) Autumn migration strategies of reed and sedge warblers. Ornis Scand 12:1–12
Chamberlain CP, Bensch S, Feng X, Åkesson S, Andersson T (2000) Stable isotopes examined across a migratory divide in Scandinavian willow warblers (Phylloscopus trochilus trochilus and Phylloscopus trochilus acredula) reflect their African winter quarters. Proc R Soc B 267:43–48
Clobert JC, Perrins CM, McCleery RH, Gosler AG (1988) Survival rate in the great tit Parus major in relation to sex, age, and immigration status. J Anim Ecol 57:287–306
Cramp S (1992) Birds of the western Palaearctic. Warblers, vol. VI. Oxford University Press, Oxford
Curry-Lindahl K (1981) Bird migration in Africa, vol. 1. Academic Press, London
De Roo A, Deheegher J (1969) Ecology of the great reed warbler, Acrocephalus arundinaceus (L.), wintering in the southern Congo savannah. Gerfaut 59:260–275
Greenwood PJ, Harvey PH (1982) The natal and breeding dispersal of birds. Annu Rev Ecol Syst 13:1–21
Hanmer DB (1986) Migrant Palearctic passerines at Nchalo, Malawi. Safring News 15:19–28
Hansson B, Bensch S, Hasselquist D, Nielsen B (2002) Restricted dispersal in a long-distance migrant bird with patchy distribution, the great reed warbler. Oecologia 130:536–542
Hedenström A, Bensch S, Haselquist D, Lockwood M, Ottosson U (1993) Migration, stopover and moult of the great reed warbler Acrocephalus arundinaceus in Ghana, West Africa. Ibis 135:177–180
Hobson KA (1999) Tracing origins and migration of wildlife using stable isotopes: a review. Oecologia 120:314–326
Hobson KA (2005) Using stable isotopes to trace long-distance dispersal in birds and other taxa. Divers Distrib 11:157–164
Hobson KA, Wassenaar LI (1997) Linking breeding and wintering grounds of neotropical migrants using stable H isotopic analysis of feathers. Oecologia 109:142–148
Holmes RT, Sherry TW (1992) Site fidelity of migratory warblers in temperate breeding and Neoptropical wintering areas: implications for population dynamics, habitat selection, and conservarion. In: Hagan JM III, Johnstone DW (eds) Ecology and conservation of Neotropical migrant land birds. Smithsonian Institution Press, Washington, pp 563–575
Kelly JF (2000) Stable isotopes of carbon and nitrogen in the study of avian and mammalian trophic ecology. Can J Zool 78:1–27
King JMB, Hutchinson JMC (2001) Site fidelity and recurrence of some migrant bird species in the Gambia. Ring Migr 20:292–302
Koch PL, Heisinger J, Moss C, Carlson R, Fogel ML, Behrensmeyer AK (1995) Isotopic tracking of change in diet and habitat use in African elephants. Science 267:1340–1343
Lajtha K, Michener RH (1994) Stable isotopes in ecology and environmental science. Blackwell Scientific, Oxford, UK
Langin MK, Reudink WM, Marra PP, Norris DR, Kyser TK, Ratcliffe ML (2007) Hydrogen isotopic variation in migratory bird tissues of known origin: implications for geographic assignment. Oecologia 152:449–457
Lessells CM, Boag PT (1987) Unrepeatable repeatabilities: a common mistake. Auk 104:116–121
Minagawa M, Wada E (1984) Stepwise enrichment of 15N along food chains: Further evidence and the relation between δ15N and animal age. Geochimica et Cosmochimica Acta 48:1135–1140
Neto JM, Newton J, Gosler AG, Perrins CM (2006) Using stable isotope analysis to determine the winter moult extent in migratory birds: the complex moult of Savi’s warblers Locustella luscinioides. J Avian Biol 37:117–124
Nisbet CT, Medway L (1972) Dispersion, population ecology and migration of eastern great reed warblers, Acrocephalus orientalis, overwintering in Malaysia. Ibis 114:451–494
Pärt T (1994) Male philopatry confers a mating advantage in the migratory collard flycatcher, Ficedula albicollis. Anim Behav 48:401–409
Salewski W, Bairlein F, Leisler B (2000) Recurrence of some Palearctic migrant species in West Africa. Ring Migr 20:29–30
Smith BN, Epstein S (1971) Two categories of 13C/12C ratios in higher plants. Plant Physiol 47:380–384
Wassenaar LI, Hobson KA (2000) Stable-carbon and hydrogen isotope ratios reveal breeding origins of redwinged blackbirds. Ecol Appl 10:911–916
Wassenaar LI, Hobson KA (2003) Comparative equilibration and online echnique for determination of non-exchangeable hydrogen of keratins for use in animal migration studies. Isotopes Environ Health Stud 39:211–217
Yohannes E, Hobson KA, Pearson DJ (2007) Feather stable-isotope profiles reveal stopover habitat selection and site fidelity in nine migratory species moving through sub-Saharan Africa. J Avian Biol 38:347–355
Acknowledgments
We thank Kenji Adachi for kind assistance in the laboratory. This study was partly supported by the Max Planck Society and NSF Grant DBI-0116203. δ13C and δ15N analyses were conducted at School of Biological Sciences, Washington State University. We thank two anonymous referees, Bern Leisler and Gerhard Nikolaus.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Bairlein.
Rights and permissions
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yohannes, E., Bensch, S. & Lee, R. Philopatry of winter moult area in migratory Great Reed Warblers Acrocephalus arundinaceus demonstrated by stable isotope profiles. J Ornithol 149, 261–265 (2008). https://doi.org/10.1007/s10336-007-0271-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-007-0271-9