Abstract
Conservation of migratory birds requires knowledge of breeding and nonbreeding ranges and the connections between them. European turtle doves (Streptopelia turtur) are Palearctic-African long-distance migrants with wintering areas in the Sub-Saharan belt that are classed as vulnerable due to strong population declines. However, detailed non-breeding locations of individuals from different migratory flyways are unknown. To identify wintering regions of turtle doves, we measured stable isotopes of feathers grown on the wintering grounds and used a dual-isotope (hydrogen (δ2Hf) and carbon (δ13Cf)) probabilistic assignment to analyse origins of individuals migrating through the western and central/eastern flyways. The most probable wintering areas for turtle dove samples from both flyways were in the western and central Sub-Sahara. However, we found differences in δ2Hf and δ13Cf values between turtle doves following different migratory routes (western vs central/eastern flyway). This result suggests a higher likelihood of origins in the central Sub-Sahara for central and eastern migrants, while turtle doves using the western flyway originated primarily in the western Sub-Sahara, highlighting the importance of both regions for the future conservation of turtle doves from European breeding populations. The establishment of migratory connectivity of populations requires sampling from birds from the European as well as Asian continent; however, we provide important results that can be used to test hypotheses regarding population declines resulting from factors experienced over the full annual cycle for some populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Migratory birds occupy geographically disparate regions during different parts of the annual cycle, and long-distance migrants typically spend the majority of their life away from their breeding sites (Kelly et al. 2005). Nonetheless, most studies have focused on factors affecting conditions during the breeding period, but research to understand how events outside the breeding season impact survival of migrants are still insufficient (e.g., Prochàzka et al. 2008; Morrison et al. 2013; Briedis et al. 2018; Tobolka et al. 2018). Therefore, research into the identification of areas used during the non-breeding period and the spatial linkages between breeding and non-breeding grounds, or 'migratory connectivity', is of crucial importance for the effective conservation of migratory birds (Prochàzka et al. 2008).
European turtle doves (Streptopelia turtur, subspecies S. t. turtur, henceforth, turtle doves) are the only long-distance migrating species of European breeding columbids and winter in the African Sahel between 10 and 20° N (Glutz von Blotzheim 1980; Geroudet 1983; Cramp 1985). Turtle doves have undergone a rapid and steep decline of ~ 80% across the European breeding range since 1980, and the species is currently listed as ‘vulnerable’ on the IUCN’s red list (Fisher et al. 2018; PECBMS 2021). Agricultural intensification in Europe, which includes the removal of hedges and extensive use of agro-chemicals, may lead to increased predation risk, a shortened breeding season, and decreased productivity (i.e., lower number of breeding attempts and fledged young per season), is suspected to be the main driver of the turtle dove decline (Browne and Aebischer 2004; Browne et al. 2005). Environmental conditions experienced on migration and on the non-breeding grounds can also affect the individual physical condition and population dynamics of migratory avian species (e.g., sedge warbler Acrocephalus schoenobaenus, Peach et al. 1990; white stork Ciconia ciconia, Kanyamibwa et al. 1990; Schaub et al. 2005; Baillie and Peach 1992; Robson and Barriocanal 2011; European sand martin Riparia riparia, Szép 1995; turtle dove, Eraud et al. 2009). Unsustainable legal and illegal hunting activities along the migration routes are further contributing to the turtle dove decline (Fisher et al. 2018; Lormée et al. 2020; Moreno-Zarate et al. 2021), with eight countries in the EU-27 reporting hunting bags totaling over 1.4 million birds (Hirschfeld et al. 2019). Furthermore, turtle doves face multiple threats on the Sahelian non-breeding grounds (henceforth winter grounds), including reduced survival resulting from food shortages, scarcity of roosting sites and freshwater, as well as an increased human disturbance (e.g., agricultural intensification, hunting; Zwarts et al. 2009). Therefore, identifying the main wintering areas of turtle dove populations is important in order to develop appropriate management and conservation strategies over the full annual cycle (Hobson et al. 2009a; Eraud et al. 2013; Hanane 2017).
Turtle doves use three main migratory flyways (western, central, and eastern) to move between breeding and wintering grounds with regular mixing between the central and eastern routes but no or limited mixing between western and other routes (Marx et al. 2016). Mirroring the three migratory flyways, previous studies suggested three different wintering regions in the western, central, and eastern Sub-Sahara, which may indicate the presence of strong migratory connectivity in turtle dove populations (Glutz von Blotzheim 1980; Zwarts et al. 2009). The strength of migratory connectivity has important consequences for the ecology and conservation of migratory species (see Webster et al. 2002) but it is not well described for turtle doves. Turtle dove populations using the western flyway mainly winter in western Africa, namely in Senegal, Gambia, Guinea Bissau, Conakry Guinea, Burkina Faso, and Mali (Morel 1987; Morel and Morel 1988; Carvalho and Dias 2001, 2003 according to Fisher et al. 2018; Aebischer 2002; Eraud et al. 2013; OFB 2021). Populations following the central route may winter in Sudan and Ethiopia, possibly reaching as far west as Mali, Burkina Faso, Ghana, and Nigeria (Zwarts et al. 2009; Schumm et al. 2021). Migratory movements of populations breeding in the eastern part of the European range remain poorly documented (Bankovics 2001; Fisher et al. 2018). Generally, suitable wintering habitats appear to be featured by abundant food and water availability as well as by large trees or patches of woodland as roosting sites (Zwarts et al. 2009). In addition, studies indicate that turtle doves move among several habitats during winter and move up to hundreds of kilometers between sites (Morel 1986; Eraud et al. 2013; Lormée et al. 2016; OFB 2021).
Currently, the efficacy of extrinsic markers, such as geolocators or satellite tags, to define main wintering areas of turtle dove populations is hampered by the small sample size of tracked individuals (Eraud et al. 2013: five geolocators; Lormée et al. 2016: three satellite tags; Schumm et al. 2021: 16 satellite tags; OFB 2021: 26 satellite tags; RSPB 2021: seven satellite tags). Turtle doves have been equipped with satellite transmitters and geolocators at their breeding grounds or during migration, but only a fraction of these tracked individuals reach their wintering quarters (e.g., five out of 16, Schumm et al. 2021), and few geolocators were retrieved back containing full data (five out of 64, Eraud et al. 2013). In addition, low recovery rates of ringed birds from the winter quarters (Zwarts et al. 2009; Marx et al. 2016) and difficulty in distinguishing between the subspecies S. t. turtur and S. t. arenicola on potentially shared wintering ranges complicate determining a complete picture of the wintering destinations (Hanane 2017). However, information from previous research, ringing, and tracking studies are useful to help define plausible wintering areas for other methods, including probabilistic assignment to origin using stable isotopes (Hobson et al. 2012).
In this study, we used intrinsic isotopic markers in metabolically inert feathers grown during the wintering period to identify African non-breeding origins of turtle doves sampled during migration and breeding periods in Europe. Feathers indirectly provide environmental isotopic information from the African wintering habitats where they were grown. Within a predetermined wintering range, feather isotope information can be used to assign turtle doves from different breeding or flyway origin to wintering areas (Hobson et al. 2009a). For this purpose, we assigned stable hydrogen (δ2Hf) and carbon (δ13Cf) isotope values of turtle dove feathers to multi-isotopic landscapes (“isoscapes”) of precipitation stable hydrogen (δ2Hp), and theoretical vegetation stable carbon (δ13C) in Africa (Bowen et al. 2005; Still and Powell 2010; Hobson et al. 2012). With this approach, we provide (I) a first approximation of the wintering regions used by turtle dove populations from different countries of origin in Europe, (II) an estimation for differences in δ2Hf, and δ13Cf among the western and the central/eastern flyway, and (III) an assessment of the current overlap between turtle dove winter quarters and existing protected areas.
Material and methods
Moult cycle of turtle doves
Generally, adult turtle doves start moult in July while on the breeding grounds (Demongin 2016). They renew the first to occasionally fourth primary feathers (i.e., P1–P4), then suspend moult until their arrival on the wintering areas around September, where they renew their remaining primary feathers (P5–P10). Hence, the outer primary feathers are grown on the wintering grounds (Morel 1986; Demongin 2016).
Feather sampling
Feather samples of adult turtle doves from the subspecies S. t. turtur were collected from migrating individuals, rescued birds from wildlife recovery centers, or breeding birds. Individuals were either hunted, rescued, trapped (e.g., whoosh or mist nets, cage traps), or they originated from museum (Natural History Museum Berlin) collections (Table 1). Adding samples from museum collections assumes continuity in precipitation isotope patterns through time: Feather δ2H values of samples originating from museum did not vary significantly relative to values of the remaining sample of respective countries, Germany, Italy, and Spain (t-test: t = 2.39, df = 5.46, P = 0.058). In order to increase the general sample size, and as the aim of the analysis was to provide a good first-order estimation of wintering regions vs information on a small geographic scale, we deem the inclusion of samples originating from museums valuable. Moreover, our inclusion of an appropriate error term in isotopic assignments (see below) renders our inferences conservative. Samples were collected during spring and autumn migration as well as during the breeding season in June and July (Marx et al. 2016). A small sample of approximately 1 cm2 was cut from the vane of the tip of the tenth primary feather (P10), i.e., outermost primary, for stable isotope analysis. In total, we collected 181 adult feather samples from seven European countries, 121 from the western flyway, 55 from the central/eastern flyway, and five samples without a reliable classification to one of the flyways (Table 1). The central and eastern European flyways were combined, as ringing data showed that several individuals crossed between those two flyways (Marx et al. 2016).
Stable isotope analyses
Stable isotope analyses were conducted at the Stable Isotope Laboratory of the Leibniz Institute for Zoo and Wildlife Research (IZW). Feather samples were washed with a 2:1 chloroform/methanol solution for 24 h and then dried for 48 h under a fume hood. A feather subsample of 0.55 ± 0.05 mg was placed into a tin capsule for carbon (C) stable isotope analyses. The tin capsules were combusted in an elemental analyzer (Flash EA 1112 Series, Thermo Fisher Scientific, Bremen, Germany) and measured with a Delta V-Advantage mass spectrometer (Thermo Fisher Scientific) connected continuous-flow mode.
Stable isotope values are given in the delta notation (δ) as parts per thousand (‰) deviation from the ratio of international standards (for C: VPDB). Secondary laboratory standards of known 13C/12C values of tyrosine (−24.0‰) and leucine (−30.3‰) were used for calibration and drift correction. The within-run accuracy of δ13C measurements was always better than 0.2‰ for laboratory standards. For stable hydrogen (H) analyses, 0.27 mg ± 0.1 mg were loaded into silver capsules (IVA Analysetechnik e.K., Meerbusch, Germany). Loaded samples in folded capsules were stored in 96-well microtiter plates loosely covered with the lid in order to allow exchange with ambient air moisture. Then, trays were placed in a compartment drier at 50 °C for at least 24 h to speed up equilibration and remove extra moisture. Afterwards, samples and standards were loaded into the carousel of a Zero Blank autosampler (Costech Analytical Technologies Inc., Italy) and flushed with dry helium for a minimum of 1 h. Samples were then dropped one by one into the elemental analyzer (EA; HT Elementanalysator HEKAtech GmbH, Wegberg, Germany), which operated at 1350 °C and contained a silicon carbide (Sic) tube filled halfway with glassy carbon chips and including a carbon/water trap. The obtained H2 sample pulse was then introduced into the stable isotope ratio mass spectrometer (Delta V advantage, ThermoFisher Scientific, Bremen, Germany) via an interface (Finnigan Conflo III, ThermoFisher Scientific, Bremen, Germany). Samples were analysed together with three in-house keratin standards, which had previously been calibrated to the USGS42 standard (Soto et al. 2017). Measured stable isotope values are reported in the delta notation (δ) as parts per thousand (‰) deviation from V-SMOW. The precision of δ2H measurements was always better than 2‰. We calculated the stable isotope value of the non-exchangeable portion of the hydrogen in samples based on laboratory keratin standards with known stable isotope values of the non-exchangeable portion of hydrogen: sheep wool from Sweden SWE-SHE (−111.6‰), sheep wool from Spain ESP-SHE (−61.5‰), and goat wool from Tanzania AFR-GOA (−26.4‰), standards that were calibrated directly to KHS and CBS laboratory keratin standards (−54.1 and −197.0‰, respectively).
We chose not to include nitrogen isotopic values (15 N/14 N) in our analyses. While δ15N has been regularly included in studies dealing with main diet and trophic relationships of marine or aquatic birds, as δ15N patterns there are relatively well understood and/or constant over large geographic areas (Hobson 2011), the use of δ15N measurements in tracing origins of animals, particularly terrestrial animals, is relatively rare (Hobson and Wassenaar 2019). This is because δ15N values in plant and animal tissues can vary even locally as values are heavily influenced by anthropogenic sources of nitrogen, in particular by agricultural inputs, including fertilizers, sewage, and agricultural animal waste, and by atmospheric deposition via fossil fuel burning (Cruz et al. 2012; Hobson et al. 2012; McMahon et al. 2013; Hobson and Wassenaar 2019).
Assignment to wintering areas
To delineate likely African winter areas of turtle doves, we applied a spatially-explicit multi-isotope likelihood assignment method (Royle and Rubenstein 2004; Wunder 2007; Hobson et al. 2009b). To accomplish this, and following Hobson et al. (2012), we used African isoscapes reflecting the (1) amount-weighted growing seasonal surface precipitation (δ2Hp) of Bowen et al. (2005) and (2) a theoretical spatial distribution of plant δ13C (Still and Powell 2010). We converted the δ2Hp isoscape into a feather δ2H (δ2Hf) isoscape using the calibration equation for common wood pigeons (Columba palumbus) from Hobson et al. (2009a; 4.73 + 0.78* δ2Hp) because we lacked a similar one for turtle doves. However, both species have relatively similar diets (Dunn et al. 2018) and are migratory therefore, we deemed use of this equation reasonable. To account for plant-feather δ13C isotope discrimination, we first conducted separate assignments using discrimination factors of + 1 and + 2 ‰ added to the plant δ13C isoscape (see Hobson et al. 2012) but found no difference in the spatial distribution between the resulting depictions. Because of this lack of difference and since turtle doves are entirely granivorous, whereas wood pigeons also take leaves and other plant matter (Dunn et al. 2018), we used a discrimination factor of + 1.5 ‰ to derive a feather δ13C isoscapes (δ13Cf). We then used digital range maps from BirdLife International and NatureServe (2011), which were extended southwards to about 4° N based on winter range information from tracking data, to restrict the assignments to the known turtle dove winter range by ‘clipping’ the calibrated feather (δ2Hf, δ13Cf) isoscapes.
To assess the likelihood that a georeferenced location (i.e., raster cell) within the feather isoscape of the turtle dove winter range was a potential area of origin, we used a multivariate normal probability density function (mvnpdf):
where \(fx\) represents the spatially explicit probability density function for xi indicating the geographic location of origin given a feather of unknown provenance (yi) with isotopic composition (δ2Hf, δ13Cf). Subscripts HC indicate the expected mean (μ), standard deviation (σ), and correlation (p) of δ2Hf and δ13Cf, respectively, for a feather grown at that location and k represents the number of isotopes. The estimated mean isotopic composition was estimated from raster cells in the calibrated isoscapes for δ2Hf and δ13Cf at each location (xi). Thus, the parameter μxi represents a vector of means for each location (xi) in the isoscape:
The variance–covariance matrix (|∑|) of the two-isotope matrix is represented as.
where diagonal elements represent expected variance for the given isotope and off-diagonal elements represent covariance between pairs of isotopes. We assumed that covariance among isoscapes was constant (Royle and Rubenstein 2004).
All cells in the upper 67% (i.e., 2:1 odds ratio) of the resulting probability surfaces from assignments for each individual were defined as likely (1) origins, and all others were considered unlikely (0). Thus, assignments conducted for feather samples resulted in a spatially explicit binary surface for each individual, which was summed (i.e., 'stacked') across assignments for all individuals in the sample to represent potential origins for the entire sample set but also per sampled country. Manipulation of digital files and assignment to origin analyses were conducted using several packages including 'raster' 2.5–8 (Hijmans 2016), 'mvnmle' 0.1–11.1 (Gross and Bates 2018), 'maptools' 0.9–9 (Bivand and Lewin-Koh 2019), and 'rgdal' 1.2–13 (Bivand et al. 2019) in the R statistical computing environment 3.5.3 (R Core Team 2019). As stable isotope values of δ2Hf were normally distributed (Shapiro–Wilk: W = 0.991, p = 0.326) but δ13Cf were not (Shapiro–Wilk: δ13Cf: W = 0.861, P < 0.005), we applied parametric and non-parametric statistics where appropriate.
To assess the overlap between existing protected areas as well as hunting areas and winter quarters of turtle doves according to the dual-isotope multivariate probabilistic assignment, we clipped the obtained assignment with a spatial dataset of protected areas (mix of polygons and points) from UNEP-WCMC (2021). The dataset was limited to 'internationally designated sites' (including UNESCO-MAB Biosphere Reserves and Ramsar Sites), 'nationally designated sites' (national parks, nature reserves, faunal reserves, wildlife sanctuaries, and reserves, and forest reserves), and 'bird sanctuaries'. The 'hunting areas' include the UNEP-WCMC categories hunting area, hunting zone, controlled hunting area, and hunting reserve.
Results
Wintering areas of sampled turtle doves
From the probabilistic assignment to origin analyses, wintering areas for pooled turtle dove samples were likely in the western and central Sub-Sahara. The most likely wintering sites in the western Sub-Sahara were in western Africa: Senegal, Gambia, Guinea-Bissau, Guinea, Sierra Leone, northern Ivory Coast, western Burkina Faso, south-western Mali, and in the central Sub-Sahara Togo, Benin, Nigeria, and North-Cameroon. No turtle doves were assigned to wintering areas in the eastern part of the Sub-Saharan Sahel region (Fig. 1). Similar to the results for pooled turtle dove samples, the assignments by flyway (Fig. 2) as well as by country (Online Resource 1) highlighted similar wintering areas as indicated above.
Assignment to likely wintering origin (moulting areas of winter-grown primary feathers) of European turtle doves (n = 181) sampled in seven different European countries (labeled and shaded grey) predicted from a multivariate normal probability distribution function based on tenth primary feather (P10) δ2H and δ13C isotope assignments of individual birds. Assignment probabilities of individuals (0 to 1) were summed according to the maximum value obtained in a pixel during the assignment process for the overall sample set representing the percent of individuals potentially originating from a cell in the isoscape. The assignment is restricted to a hitherto described turtle dove wintering range (outline; in red in the online version). (a) Overlap of likely wintering areas with internationally and nationally protected areas as well as bird sanctuaries, and (b) overlap with hunting areas. Information on protected and hunting areas was based on a dataset (mix of polygons and points) from UNEP-WCMC (2021)
Assignments to likely wintering origin (moulting areas of winter-grown primary feathers) of European turtle doves following (a) the western flyway (n = 121) sampled in two different European countries (grey shaded) and (b) the central/eastern flyway (n = 55) sampled in four different countries (grey shaded) predicted from a multivariate normal probability distribution function based on tenth primary feather (P10) δ2H and δ13C isotope assignments of individual birds. Assignment probabilities of individuals (0 to 1) were summed according to the maximum value obtained in a pixel during the assignment process for (a) individuals following the western flyway and (b) turtle doves following the central-eastern flyway representing the percent of individuals potentially originating from a cell in the isoscape. The assignments are restricted to a hitherto described turtle dove wintering range (outline; in red in the online version)
Differences in δ2Hf and δ13Cf values between flyways
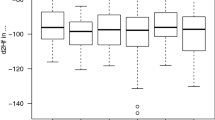
Whereas the assignment depictions (Fig. 2) do not indicate a marked difference between the wintering grounds of turtle doves following the western (samples from Spain and France) vs the central/eastern flyway (samples from Greece, Malta, Italy, and Bulgaria), comparisons of the raw δ2Hf (t-test: t = 2.77, df = 77.94, P = 0.007), and δ13Cf values (Mann–Whitney: W = 4337, P = 0.001) show differences between flyways. Feathers of turtle doves following the central/eastern migratory flyway compared to those of individuals using the western flyway had significantly higher δ2Hf (means ± SD: −60.87 ± 2.51‰ vs −68.52 ± 1.16‰, respectively) and δ13Cf (means: −18.25 ± 0.67‰ vs −20.91 ± 0.38‰) values (Fig. 3).
Boxplots for δ2Hf and δ13Cf values from the tenth primary (P10) feather samples of turtle doves moulted during the overwintering period at African winter grounds grouped by central/eastern (n = 55) and western (n = 121) flyways. The boxes represent the range in which 50% of the data occur (inter-quartile distance from 25% quartile to 75% quartile). Whiskers show extreme values, and the median is shown as a black line within the boxes. Circles highlight outliers and extend 1.5 times beyond the inter-quartile distance
Overlap of assigned wintering grounds with protected areas
International biosphere reserves and Ramsar sites overlapping with the most likely wintering grounds of turtle doves (based on the probabilistic assignment) were mainly located in Guinea and Senegal and to a smaller extent in Mali and Sierra Leone. No internationally protected areas were situated in Benin, Ivory Coast, Cameroon, and Nigeria in areas of a high probability origin (Fig. 1a). Most of the larger national protected areas and bird sanctuaries were located in the central and eastern part of our preselected area, where wintering, according to our results, is rather unlikely. Smaller national protected areas overlapped with the most likely wintering locations in many countries (e.g., Senegal, Mali, and Niger) but were also scarce or absent in others, such as Benin, Cameroon, and Ivory Coast (Fig. 1a). Some hunting areas were located in areas of the high probability of European turtle dove origins, which are among the most likely wintering grounds, in Guinea-Bissau and Mali (Fig. 1b). However, only a few hunting areas were included in the UNEP-WCMC dataset.
Discussion
The main objective of our investigation was to determine general and flyway-specific wintering regions of turtle doves sampled on European breeding areas and migration following the western and central/eastern migratory routes. Previous assessments of wintering regions and connectivity were based on a relatively small number of reported sightings, which may include discrimination errors of subspecies (S. t. turtur vs S. t. arenicola), or recaptures of ringed birds and tracked individuals. Yet, the decline of European populations of turtle doves over the past decades calls for a more thorough understanding of migratory connectivity between summer and wintering ranges (Fisher et al. 2018). Based on our dual-isotope geographic assignment, we identified regions in the western and central sub-Saharan belt to be the most likely wintering grounds of turtle doves originating from Europe.
Stable isotopes
Feather hydrogen (δ2Hf)
While assignments to the wintering origin based on deuterium values only did not show a clear difference for the different flyways (Online Resource 2), turtle doves using the western migration flyway and those using the central/eastern migration flyway differed in the raw δ2Hf values of the tenth primary feather (P10) grown on the wintering range. This potentially indicates that turtle dove populations from western vs central/eastern Europe may spend the boreal winter in different areas, for example, lower δ2Hf values in European hoopoes sampled along the central/eastern flyway suggest more easterly wintering areas for these individuals (Reichlin et al. 2013). However, the geographic precision of this assignment was limited due to large confidence intervals and a shallow slope, and the difference in deuterium values in body feathers for the longitudinal −10° to + 15° range was relatively minor (Reichlin et al. 2013).
Feather carbon (δ13Cf)
Similar to differences in δ2Hf, our results demonstrate higher raw δ13Cf values in turtle doves using the central/eastern flyway vs western flyway. The relative abundance of stable carbon isotopes depends on the relative contribution of plant biomass with contrasting photosynthetic pathways, such as Calvin cycle (C3), Hatch-Slack cycle (C4) or Crassulacean acid metabolism (CAM) (O’Leary 1981; Rubenstein and Hobson 2004), whereby C3 plants, mainly trees, and shrubs, are typically isotopically depleted in 13C in relation to 12C compared to C4 plants, which are mostly grasses (Marshall et al. 2007). While δ13Cf values may provide minor additional information concerning E–W delineations of individuals, they can indicate possible habitat segregation. For instance, regardless of the photosynthetic pathway, more xeric environments typically have higher δ13C values compared to mesic regions (Reichlin et al. 2013). Therefore, higher δ13Cf values in turtle doves using the central/eastern migration route suggest that they occupied more xeric regions than birds using the western flyway. However, differences in δ13Cf values may also reflect diet and microhabitat (Veen et al. 2014), and differences in the diet of individuals following the different migration routes might indicate different areas used for wintering with different plant species (C3 vs C4) consumed by doves. Much of the variation in δ13C values of animal tissues can be ascribed to the differential use of C4/CAM and C3 plant-based food (Lajtha and Michener 2007; Procházka et al. 2010), but there are also local habitat-specific δ13C signatures on small latitudinal gradients as a result of the water-use efficiency of the dominant C3 plant species (see Marra et al. 1998; Paxton and Moore 2015). Higher δ13Cf values in birds following the central/eastern vs the western migration route may be an indicator for winter occupancy of lower-quality habitats (Marra et al. 1998) and/or differences in the proportion of consumed food plants. While some studies analysed the diet of turtle doves on their European breeding grounds (e.g., Murton et al. 1964, 1965; Browne and Aebischer 2003; Dunn et al. 2018), very few have addressed the diet composition during the wintering period. Observations on some wintering localities indicate that rice (Oryza, C3 plant) and wild fonio (Panicum, C4 plant) may be the main food sources for wintering turtle doves (e.g., Morel 1986; Curry and Sayer 1979; Zwarts et al. 2009). Detailed studies are needed to gain a more precise picture of the dietary composition on the wintering grounds.
Differences in δ2Hf and δ13Cf values
Derived from the probabilistic assignments based on both δ2H and δ13C values (Fig. 2; based on deuterium values only, Online Resource 2), no obvious difference in the derived probability of origin during winter of individuals sampled along the western or the central/eastern flyway was found. Therefore, based solely on the assignments, we cannot conclude that the Sub-Saharan wintering range of turtle doves varies with their flyway and cannot differentiate geographic wintering ranges by flyway. Such assignments are, however, dependent on the structure of the underlying isoscape and further insight is still possible by examining each isotope individually. When comparing raw values of δ2Hf and δ13Cf, both isotopes suggest that individuals from different migratory flyways spent the winter in different Sahelian regions, had different diets, or used different habitats. Several studies already identified populations of bird species that differ in their wintering regions or habitats on the basis of isotopic analysis of feathers and used these results to estimate migratory connectivity (e.g., aquatic warbler Acrocephalus paludicola, Pain et al. 2004; reed warbler Acrocephalus scirpaceus, Procházka et al. 2008; sand martin Riparia riparia and barn swallow Hirundo rustica, Szép et al. 2009; blue swallow Hirundo atrocaerulea, Wakelin et al. 2011).
Migratory connectivity links breeding and non-breeding grounds of a species and ranges in strength from weak to strong (Webster et al. 2002). Strong connectivity occurs when most individuals from one breeding population migrate to the same wintering location or region, whereas weak connectivity occurs when individuals from a single breeding population move to several different regions to winter or from several breeding areas to a single non-breeding area (Webster et al. 2002; Rubenstein and Hobson 2004). Zwarts et al. (2009) investigated migratory connectivity of bird species based on recoveries and recaptures in Africa between 4 and 35° N. From their analysis, the west–east distribution in Africa of birds breeding in different longitudinal zones in Europe showed hardly any overlap for turtle doves, indicating strong migratory connectivity to broad regions (Zwarts et al. 2009).
Consistent with this result, isotope values of turtle doves in this study suggest a relationship between migration route (west vs central/eastern flyway) and African winter moulting grounds (western part of the Sahel vs central part of the Sahel, respectively), but this was not supported by the assignment to origin analyses, which could be interpreted as being contradictory. Although differences in feather stable isotope values between flyways were significant, they are small relative to the variation in the associated feather isoscapes. Despite the heterogeneity of sample origin regarding collection period and sample location (see Table 1), based on the moult cycle, all sampled tenth primary feathers had been grown during the previous winter and, therefore, reliably contained the isotopic signature of wintering grounds. However, sample sizes for western (n = 121) and central/eastern flyway (n = 55) were not equally distributed, which could have caused a bias towards the western part of the Sahara. In general, there has been a strong bias of studies towards turtle doves using the western flyway, whereas detailed knowledge of turtle doves migrating via the central/eastern flyway as well as a stopover and wintering sites of central and eastern turtle dove populations remains limited (Bankovics 2001; Fisher et al. 2018). Therefore, our findings for individuals using the central/eastern flyway, even if they originate from a comparably small sample size, add valuable new information.
The use of stable isotopes and museum samples present several challenges for assessing turtle dove migratory connectivity. Our analysis could potentially have been more precise if individuals using the central and eastern flyways were examined separately. However, as tracking and ringing data indicate a loop-migration pattern between the central and eastern flyway (Marx et al. 2016; Schumm et al. 2021), separate analyses potentially could result in incorrect assignment to a flyway for some individuals. Also, for individuals with breeding sites in western Europe, such as France, it was described that individuals migrate more easterly during spring than during autumn (Eraud et al. 2013). To our knowledge, however, no switch of flyways, i.e., from western to central flyway, has been observed so far. The inclusion of museum specimens (1890 to 2014; Table 1) could also potentially bias our results due to changes in distributional patterns in breeding and wintering areas and hotspots of natal origin because declines are expected to cause range retractions towards available and optimal habitats (Thomas et al. 2008; Sirami et al. 2009; Burgess et al. 2020). Contraction towards high-quality habitats may be reflected by varying levels of observed decline across the European breeding area, e.g., a strong population decline of 97% (1967 to 2015) in the UK or > 90% (1984 to 2015) in the Netherlands, compared to less pronounced declines of 37% (1996 to 2018) in Spain or of 54% (1998 to 2015) in Austria (see De Vries et al. 2021). Furthermore, habitat modifications through time resulting from agricultural expansion may result in changes to the δ13C isoscape or diet potentially influencing the isotope values between modern and historic samples (Lemenih et al. 2005; English et al. 2018; Arias-Ortiz et al. 2021). However, feather isotope values of samples from museums did not vary significantly relative to region-specific samples. Finally, our aim was to provide a first-order estimation of wintering regions and not an assignment on a small geographic scale or analysing changes over time, so we deem the use of museums samples reasonable.
Another study that investigated breeding ground provenance of migrating turtle doves, including some of the sampled individuals from this study, showed relatively coarse and broad possible distribution ranges using δ2Hf only, and no spatially precise breeding localities could be assigned (Marx et al. 2020). Therefore, a precise estimation of the connectivity between breeding and wintering areas was not possible in this study, as we do not know the exact breeding areas of individuals sampled during migration. Nevertheless, the use of stable isotopes can provide insight into the migratory connectivity of species, especially when involving the use of informed priors in a Bayesian framework (see Hobson and Wassenaar 2019). Combining probabilistic information based on other methods, such as analysis of ringing or tracking data, can indeed inform evidence-based, long-term, and effective conservation measures for threatened species. Such a combined approach is urgently needed to confirm the possible strong migratory connectivity for turtle doves, as turtle doves originating from different breeding grounds may be subject to several different or variable levels of similar threats across the full annual cycle. For example, a breeding population from one country can experience little or no decline while another is decreasing drastically due to factors experienced at different stopover or wintering sites (e.g., habitat loss, unsustainable harvesting; Weber et al. 1999; Runge et al. 2014).
Conclusions and perspectives
Our results highlight the potential importance of the western and central Sub-Sahara as a wintering region for turtle doves migrating through western and central Europe and thus partly support previously described wintering ranges (see Glutz von Blotzheim 1980; Carvalho and Dias 2001, 2003; Aebischer 2002). However, this and previous studies focused on the analyses of flyways within the European-African migration system (Zwarts et al. 2009; Marx et al. 2016), and little information is available from the eastern Sahel (Zwarts et al. 2009). While our results did not assign any turtle doves to the eastern part of the Sahel, some sightings of turtle doves (e.g., Sudan: Hartmann 1863; Nikolaus 1987) suggest that this region also hosts wintering individuals. Hence, it is possible that the eastern sub-Saharan region is important for turtle dove populations breeding in Asia (e.g., Russia, Kazakhstan, Turkmenistan, Uzbekistan; BirdLife International 2021), being part of the Asian-African migratory system (Cramp 1985), or for birds from the central/eastern European flyway, breeding farther east than turtle doves sampled in this study, e.g., Ukraine, Turkey. A longitudinal gradient of non-breeding African areas, with western and central regions being occupied by European populations and the eastern region being occupied by Asian populations, has also been observed in other migrating species whose breeding distribution ranges from western Europe to middle and eastern Asia (Trierweiler et al. 2014; Sarà et al. 2019). However, to confirm whether Asian turtle dove populations winter in eastern sub-Saharan Africa, stable isotope studies are similar to our approach and ideally combined with tracking studies (e.g., Jiguet et al. 2019; Monti et al. 2021) are needed to investigate their flyways and wintering destinations.
The present study provides a good first-order estimation of wintering regions of turtle doves from European populations but still has some limitations due to potential winter movements of turtle doves (Eraud et al. 2013; Lormée et al. 2016) that could not be accounted for. Furthermore, our analysis did not provide information on inter-annual wintering site fidelity, which may be important for the planning and maintenance of protected areas. For instance, we show that based on the probabilistic wintering ranges, large parts of the most likely wintering grounds do not overlap with the protected area network in Africa. Turtle dove conservation management should consider hunting regulations over the species’ entire range. Whereas much attention has been given to the impact of illegal hunting during migration in Europe on population declines, very little consideration has been given to hunting in African wintering areas (Hirschfeld et al. 2019; Lormée et al. 2020), where the resources to enforce hunting laws are much more limited. It is a common practice to shoot turtle doves at roost and drinking sites in some countries, e.g., Senegal and Mali. These hunting activities at roosting sites are likely to affect survival not solely through direct mortality via shooting but also by scaring away turtle doves from safe and suitable feeding and roosting sites (Zwarts et al. 2009).
In general, defining wintering regions is important, as the causes of population decline in European-breeding migrants are associated with the region and habitats in which they winter (Ockedon et al. 2012). A significant part of the variance in the annual survival of turtle doves was explained by environmental conditions encountered by birds on their wintering grounds (Eraud et al. 2009). Nevertheless, a substantial knowledge gap on conditions and threats that turtle doves face on the wintering grounds remains. This gap needs to be filled urgently in order to understand the factors leading to the turtle dove decline (Fisher et al. 2018). Wintering conditions are likely to deteriorate further in the future, as in the sub-Saharan region where agricultural landscapes are changing rapidly (Cour 2001; Sissoko et al. 2011; Walther 2016). Increasing human pressure in this region has resulted in a reduction of preferred habitat (i.e., woody vegetation) and migratory birds have suffered particularly severe declines (Walther 2016). Turtle doves are susceptible to previous and ongoing changes, such as increased cultivation of the Sahel and Sudan zone, overgrazing and cutting of trees, overuse of pesticides, suppression of woodland within farmland, and the homogenization of cropland (Lutz 2007; Fisher et al. 2018; Mansouri et al. 2020). Moreover, similar to other migratory species, turtle doves are particularly at-risk due to global climate change, as they must adapt their breeding and migration timing to asynchronous changes in suitable conditions across broad, spatiotemporal scales (Fraser et al. 2019).
Availability of data and material
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Change history
13 September 2023
A Correction to this paper has been published: https://doi.org/10.1007/s10344-023-01724-9
References
Aebischer NJ (2002) The European Turtle Dove Streptopelia turtur. In: Wernham CV, Toms MP, Marchant JH, Clark JA, Siriwardena GM, Baillie SR (eds) The migration atlas: movements of the birds of Britain and Ireland. T and AD Poyser, London, pp 420–422
Arias-Ortiz A, Masqué P, Glass L et al (2021) Losses of soil organic carbon with deforestation in mangroves of Madagascar. Ecosystems 24:1–19. https://doi.org/10.1007/s10021-020-00500-z
Baillie SR, Peach WJ (1992) Population limitation in Palaearctic-African migrant passerines. Ibis 134:120–132. https://doi.org/10.1111/j.1474-919X.1992.tb04742.x
Bankovics A (2001) The migration of wood pigeon (Columba palumbus) and turtle dove (Streptopelia turtur) in Hungary. Naturzale 16:83–93
BirdLife International & Nature Serve (2011) Bird species distribution maps of the world. Cambridge, UK and Nature Serve, Arlington, USA: BirdLife International
BirdLife International (2021) IUCN Red list for birds. Downloaded from http://www.birdlife.org. Accessed 19 Feb 2021
Bivand RS, Keitt T, Rowlingson B (2019) rgdal: bindings for the ‘geospatial’ data abstraction library. R package version 1.4–7. https://CRAN.R-project.org/package=rgdal
Bivand RS, Lewin-Koh N (2019) R-Package maptools: tools for reading and handling spatial objects. https://cran.r-project.org/web/packages/maptools
Bowen GJ, Wassenaar LI, Hobson KA (2005) Global application of stable hydrogen and oxygen isotopes to wildlife forensics. Oecologia 143:337–348. https://doi.org/10.1007/s00442-004-1813-y
Briedis M, Krist M, Král M, Voigt CC, Adamík P (2018) Linking events throughout the annual cycle in a migratory bird—non-breeding period buffers accumulation of carry-over effects. Behav Ecol Sociobiol 72:93. https://doi.org/10.1007/s00265-018-2509-3
Browne SJ, Aebischer NJ (2003) Habitat use, foraging ecology and diet of turtle doves Streptopelia turtur in Britain. Ibis 145:572–582. https://doi.org/10.1046/j.1474-919X.2003.00185.x
Browne SJ, Aebischer NJ (2004) Temporal changes in the breeding ecology of turtle doves Streptopelia turtur in Britain, and implications for conservation. Ibis 146:125–137. https://doi.org/10.1111/j.1474-919X.2004.00235.x
Browne SJ, Aebischer NJ, Crick HQP (2005) Breeding ecology of turtle doves Streptopelia turtur in Britain during the period 1941–2000: an analysis of BTO nest record cards. Bird Study 52:1–9. https://doi.org/10.1080/00063650509461368
Burgess MD, Eaton MA, Gregory RD (2020) A review of spatial patterns across species ranges to aid the targeting of conservation interventions. Biol Conserv 251:108755. https://doi.org/10.1016/j.biocon.2020.108755
Carvalho M, Dias S (2001) Análise dos quadros de caça de columbídeos na região de Cape (Guiné-Bissau) – contributo para a suagestão cinegética. In Livro de resumos do III Congresso Ornitologia da Sociedade Portuguesa para o Estudo das Aves. SPEA, Castelo Branco 23
Carvalho M, Dias S (2003) Game Columbidae in Guinea- Bissau. XXVIth Congress of the International Union of Game Biologists Integrating Wildlife with People. Braga, Portugal. Abstract book 67
Cour JM (2001) The Sahel in West Africa: countries in transition to a full market economy. Global Environ Chang 11:31–47. https://doi.org/10.1016/S0959-3780(00)00043-1
Cramp S (1985) Handbook of the birds of Europe, the Middle East and North Africa, vol IV. Terns to Woodpeckers. Oxford University Press, Oxford 353–363
Cruz VC, Araújo PC, Sartori JR, Pezzato AC, Denadai JC, Polycarpo GV, Zanetti LH, Ducatti C (2012) Poultry offal meal in chicken: traceability using the technique of carbon (13 C/12 C) and nitrogen (15 N/14 N) stable isotopes. Poult Sci 91(2):478–486. https://doi.org/10.3382/ps.2011-01512
Curry J, Sayer JA (1979) The inundation zone of the Niger as an environment for Palaearctic migrants. Ibis 121:20–40. https://doi.org/10.1111/j.1474-919X.1979.tb05012.x
Demongin L (2016) Identification guide to birds in the hand. Privately published, Beauregard-Vendon, France 184–185
De Vries EHJ, Foppen RPB, van der Jeugd H, Jongejans E (2021) Searching for the causes of decline in the Dutch population of European turtle doves (Streptopelia turtur). Ibis Advance Online Publication. https://doi.org/10.1111/ibi.13031
Dunn J, Stockdale J, Moorhouse-Gann R, Mccubbin A, Hipperson H, Morris A, Grice P, Symondson W (2018) The decline of the turtle dove: dietary associations with body condition and competition with other columbids analysed using high-throughput sequencing. Mol Ecol 27:3386–3407. https://doi.org/10.1111/mec.14766
English PA, Green DJ, Nocera JJ (2018) Stable isotopes from museum specimens may provide evidence of long-term change in the trophic ecology of a migratory aerial insectivore. Front Ecol Evol 6:14. https://doi.org/10.3389/fevo.2018.00014
Eraud C, Boutin JM, Rivière M, Le Brun J, Barbraud C, Lormée H (2009) Survival of turtle doves Streptopelia turtur in relation to western Africa environmental conditions. Ibis 151:186–190. https://doi.org/10.1111/j.1474-919X.2008.00876.x
Eraud C, Rivière M, Lormée H, Fox JW, Ducamp JJ, Boutin JM (2013) Migration routes and staging areas of trans-Saharan turtle doves appraised from light-level geolocators. PLoS One 8:e59396. https://doi.org/10.1371/journal.pone.0059396
Fisher I, Ashpole J, Scallan D, Carboneras C, Proud T (compilers) (2018) International single species action plan for the conservation of the European turtle-dove Streptopelia turtur (2018 to 2028). European Commission Technical Report xxx-2018
Fraser KC, Shave A, de Greef E, Siegrist J, Garroway CJ (2019) Individual variability in migration timing can explain long-term, population-level advances in a Songbird. Front Ecol Evol 7:324. https://doi.org/10.3389/fevo.2019.00324
Geroudet P (1983) Limicoles, Gangas et Pigeons d’Europe, vol 2. Delachaux et Niestlé, Neuchâtel
Glutz von Blotzheim UN (1980) Handbuch der Vögel Mitteleuropas. Akademische Verlagsgesellschaft, Wiesbaden
Gross K, Bates D (2018) mvnmle: ML estimation for multivariate normal data with missing values. R package version 0.1–11 https://CRAN.R-project.org/package=mvnmle
Hanane S (2017) The European turtle-dove Streptopelia turtur in Northwest Africa: a review of current knowledge and priorities for future research. Ardeola 64:273–287. https://doi.org/10.13157/arla.64.2.2017.rp1
Hartmann R (1863) Ornithologische Reiseskizzen Aus Nordost-Afrika J Ornithol 11:299–320
Hijmans RJ (2016) raster: geographic data analysis and modeling. https://cran.r-project.org/web/packages/raster
Hirschfeld A, Attard G, Scott L (2019) Bird hunting in Europe: an analysis of bag figures and the potential impact on the conservation of threatened species. Br Birds 112:153–166
Hobson KA, Lormée H, Van Wilgenburg SL, Wassenaar LI, Boutin JM (2009a) Stable isotopes (δD) delineate the origins and migratory connectivity of harvested animals: the case of European woodpigeons. J Appl Ecol 46:572–581. https://doi.org/10.1111/j.1365-2664.2009.01651.x
Hobson KA, Wunder MB, Van Wilgenburg SL, Clark RG, Wassenaar LI (2009b) A method for investigating population declines of migratory birds using stable isotopes: origins of harvested lesser scaup in North America. PLoS One 4:e7915. https://doi.org/10.1371/journal.pone.0007915
Hobson KA (2011) Isotopic Ornithology: a Perspective J Ornithol 152(S1):49–66. https://doi.org/10.1007/s10336-011-0653-x
Hobson KA, Van Wilgenburg SL, Wassenaar LI, Powell RL, Still CJ, Craine JM (2012) A multi isotope (d13C, d15N, d2H) feather isoscape to assign Afrotropical migrant birds to origins. Ecosphere 3(5):44. https://doi.org/10.1890/ES12-00018.1
Hobson KA, Wassenaar LI (2019) Tracking animal migration with stable isotopes. 2nd Edition. Academic Press
Jiguet F, Robert A, Lorrillière R et al (2019) Unravelling migration connectivity reveals unsustainable hunting of the declining ortolan bunting. Sci Adv 5(5):eaau2642 https://doi.org/10.1126/sciadv.aau2642
Kanyamibwa S, Schierer A, Pradel R, Lebreton JD (1990) Changes in adult annual survival rates in a western European population of the white stork Ciconia ciconia. Ibis 132:27–35. https://doi.org/10.1111/j.1474-919X.1990.tb01013.x
Kelly JF, Ruegg KC, Smith TB (2005) Combining isotopic and genetic markers to identify breeding origins of migrant birds. Ecol Appl 15(5):1487–1494. https://doi.org/10.1890/04-1704
Lajtha K, Michener RH (2007) Stable isotopes in ecology and environmental science, 2nd edn. Blackwell, Oxford
Lemenih M, Karltun E, Olsson M (2005) Soil organic matter dynamics after deforestation along a farm field chronosequence in southern highlands of Ethiopia. Agric Ecosyst Environ 109:9–19. https://doi.org/10.1016/j.agee.2005.02.015
Lormée H, Barbraud C, Peach W, Carboneras C, Lebreton JD, Moreno-Zarate L, Bacon L, Eraud C (2020) Assessing the sustainability of harvest of the European turtle-dove along the European western flyway. Bird Conserv Int 30:506–521. https://doi.org/10.1017/S0959270919000479
Lormée H, Boutin JM, Pinaud D, Bidault H, Eraud C (2016) Turtle dove Streptopelia turtur migration routes and wintering areas revealed using satellite telemetry. Bird Study 63:425–429. https://doi.org/10.1080/00063657.2016.1185086
Lutz M (2007) Management plan for turtle-dove (Streptopelia turtur) 2007–2009. European Commission, Technical Report 007–2007
Mansouri I, Ousaaid D, Squalli W, Sqalli H, Ghadraoui LE, Dakki M (2020) The turtle dove (Streptopelia turtur) in Midelt plain, Morocco: nesting preferences and breeding success versus the impact of predation and agricultural practices. J Anim Behav Biometeorol 8:206–214. https://doi.org/10.31893/jabb.20027
Marra P, Hobson K, Holmes R (1998) Linking winter and summer events in a migratory bird by using stable-carbon isotopes. Science 282:1884–1886. https://doi.org/10.1126/science.282.5395.1884
Marshall JD, Brooks R, Lajtha K (2007) Sources of variation in the stable isotopic composition of plants. In: Michener R, Lajtha K (eds) Stable isotopes in ecology and environmental science, 2nd edn. Blackwell Publishing, Oxford 22–60
Marx M, Korner-Nievergelt F, Quillfeldt P (2016) Analysis of ring recoveries of European turtle doves Streptopelia turtur - flyways, migration timing and origin areas of hunted birds. Acta Ornithol 51:55–70. https://doi.org/10.3161/00016454AO2016.51.1.005
Marx M, Rocha G, Zehtindjiev P et al (2020) Using stable isotopes to assess population connectivity in the declining European turtle dove (Streptopelia turtur). Conserv Sci Pract 2:e152. https://doi.org/10.1111/csp2.152
McMahon KW, Hamady LL, Thorrold SR (2013) A review of ecogeochemistry approaches to estimating movements of marine animals. Limnol Oceanogr 58(2):697–714. https://doi.org/10.4319/lo.2013.58.2.0697
Monti F, Robert A, Dominici JM et al (2021) Using GPS tracking and stable multi-isotopes for estimating habitat use and winter range in Palearctic ospreys. Oecologia 195:655–666. https://doi.org/10.1007/s00442-021-04855-5
Morel MY (1986) Mue et engraissement de la Tourterelle des Bois Streptopelia turtur, dans une steppe arbustive du nord Sénégal, région de Richard-Toll. Alauda 54:121–137
Morel MY (1987) La Tourterelle des Bois, Streptopelia turtur, dans l’ouest africain: mouvements migratoires et regime alimentaire. Malimbus 9:23–42
Morel GJ, Morel MY (1988) Nouvelles données sur l’hivernage de la tourterelle des bois, Streptopelia turtur, en Afrique de l’Ouest: Nord de la Guinée. Alauda 56(2):85–91
Moreno-Zarate L, Arroyo B, Peach W (2021) Effectiveness of hunting regulations for the conservation of a globally-threatened species: the case of the European turtle-dove in Spain. Biol Conserv 256:109067. https://doi.org/10.1016/j.biocon.2021.109067
Morrison CA, Robinson RA, Clark JA, Risely K, Gill JA (2013) Recent population declines in Afro-Palaearctic migratory birds: the influence of breeding and non-breeding seasons. Divers Distrib 19:1051–1058. https://doi.org/10.1111/ddi.12084
Murton R, Westwood N, Isaacson A (1964) The feeding habits of the Woodpigeon Columba palumbus, Stock Dove C. oenas and Turtle Dove Streptopelia turtur. Ibis 106:174–188. https://doi.org/10.1111/j.1474-919X.1964.tb03694.x
Murton RK, Westwood NJ, Isaacson AJ (1965) Russian oberservations by G. N. Likhachev on the diet of stock doves Columba oenas and turtle doves Streptopelia turtur. Ibis 107(2):254–256. https://doi.org/10.1111/j.1474-919X.1965.tb07303.x
Nikolaus G (1987) Distribution atlas of Sudan’s birds with notes on habitat and status. Bonner Zoologische Monographien 25:1–322
Ockedon N, Hewson CM, Johnston A, Atkinson PW (2012) Declines in British-breeding populations of Afro-Palaearctic migrant birds are linked to bioclimatic wintering zone in Africa, possibly via constraints on arrival time advancement. Bird Study 59(2):111–125. https://doi.org/10.1080/00063657.2011.645798
OFB (2021) Office Français de la Biodiversité. La tourterelle des bois Streptopelia turtur. Online at: http://turtledoveresearch.com/fr/. Accessed 18 Feb 2021
O’Leary MH (1981) Carbon isotope fractionation in plants. Phytochemistry 20:553–567. https://doi.org/10.1016/0031-9422(81)85134-5
Pain D, Green R, Giebetaing B, Kozulin A, Poluda A, Ottosson U, Flade M, Hilton G (2004) Using stable isotopes to investigate migratory connectivity of the globally threatened Aquatic Warbler Acrocephalus paludicola. Oecologia 138:168–174. https://doi.org/10.1007/s00442-003-1416-z
Paxton KL, Moore FR (2015) Carry-over effects of winter habitat quality on en route timing and condition of a migratory passerine during spring migration. J Avian Biol 46:495–506. https://doi.org/10.1111/jav.00614
Peach WJ, Baillie SR, Underhill LG (1990) Survival of British Sedge Warblers (Acrocephalus schoenobaenus) in relation to West African rainfall. Ibis 133:300–305. https://doi.org/10.1111/j.1474-919X.1991.tb04573.x
PECBMS (2021) Population trends of common European breeding birds. Online at: https://pecbms.info/trends-and-indicators/species-trends/species/streptopelia-turtur/. Accessed 20 Feb 2021
Procházka P, Hobson K, Karcza Z, Kralj J (2008) Birds of a feather winter together: migratory connectivity in the Reed Warbler Acrocephalus scirpaceus. J Ornithol 149:141–150. https://doi.org/10.1007/s10336-007-0250-1
Procházka P, Reif J, Horák D, Klvana P, Lee RW, Yohannes E (2010) Using stable isotopes to trace resource acquisition and trophic position in four Afrotropical birds with different diets. Ostrich 81(3):273–275. https://doi.org/10.2989/00306525.2010.519889
R Core Team (2019) R: A language and environment for statistical computing. Vienna, Austria: The R Foundation for Statistical Computing. http://www.r-project.org/index.html
Reichlin T, Hobson K, Van Wilgenburg S, Schaub M, Wassenaar L, Martín-Vivaldi M, Arlettaz R, Jenni L (2013) Conservation through connectivity: can isotopic gradients in Africa reveal winter quarters of a migratory bird? Oecologia 171:591–600. https://doi.org/10.1007/s00442-012-2418-5
Robson D, Barriocanal C (2011) Ecological conditions in wintering and passage areas as determinants of timing of spring migration in trans-Saharan migratory birds. J Anim Ecol 80:320–331. https://doi.org/10.1111/j.1365-2656.2010.01772.x
Royle JA, Rubenstein DR (2004) The role of species abundance in determining breeding origins of migratory birds with stable isotopes. Ecol Appl 14:1780–1788. https://doi.org/10.1890/04-0175
RSPB (2021) The Royal Society for the protection of birds: tracking turtle doves. Online at: https://www.rspb.org.uk/our-work/conservation/satellite-tracking-birds/tracking-turtle-doves/. Accessed 18 Feb 2021
Rubenstein DR, Hobson KA (2004) From birds to butterflies: animal movement patterns and stable isotopes. Trends Ecol Evol 19(5):256–263. https://doi.org/10.1016/j.tree.2004.03.017
Runge CA, Martin TG, Possingham HP, Willis SG, Fuller RA (2014) Conserving mobile species. Front Ecol Environ 14(7):395–402. https://doi.org/10.1890/130237
Sarà M, Bondì S, Bermejo A et al (2019) Broad-front migration leads to strong migratory connectivity in the lesser kestrel (Falco naumanni). J Biogeogr 46(12):2663–2677. https://doi.org/10.1111/jbi.13713
Schaub M, Wojciech K, Köppen U (2005) Variation of primary production during winter indices synchrony in survival rates in migratory white storks Ciconia ciconia. J Anim Ecol 74:656–666. https://doi.org/10.1111/j.1365-2656.2005.00961.x
Schumm YR, Metzger B, Neuling E, Austad M, Galea N, Barbara N, Quillfeldt P (2021) Year-round spatial distribution and migration phenology of a rapidly declining trans-Saharan migrant—evidence of winter movements and breeding site fidelity in European turtle doves. Behav Ecol Sociobiol 75:152. https://doi.org/10.1007/s00265-021-03082-5
Sirami C, Brotons L, Martin JL (2009) Do bird spatial distribution patterns reflect population trends in changing landscapes? Landscape Ecol 24:893–906. https://doi.org/10.1007/s10980-009-9365-5
Sissoko K, van Keulen H, Verhagen J, Tekken V, Battaglini A (2011) Agriculture, livelihoods and climate change in the West African Sahel. Reg Environ Change 11:119–125. https://doi.org/10.1007/s10113-010-0164-y
Soto DX, Koehler G, Wassenaar LI, Hobson KA (2017) Re-evaluation of the hydrogen stable isotopic composition of keratin calibration standards for wildlife and forensic science applications. Rapid Commun Mass Spectrom 31:1193–1203. https://doi.org/10.1002/rcm.7893
Still CJ, Powell RL (2010) Continental-scale distributions of vegetation stable carbon isotope ratios. In: West JB, Bowen GJ, Dawson TE, Tu KP (eds) Isoscapes: understanding movements, pattern and process on Earth through isotope mapping. Springer, New York, pp 179–194
Szép T (1995) Relationship between West African rainfall and the survival of central European Sand Martins Riparia riparia. Ibis 137:162168. https://doi.org/10.1111/j.1474-919X.1995.tb03235.x
Szép T, Hobson KA, Vallner J, Piper SE, Kovács B, Szabó ZD, Møller AP (2009) Comparison of trace element and stable isotope approaches to the study of migratory connectivity: an example using two hirundine species breeding in Europe and wintering in Africa. J Ornithol 150:621–636. https://doi.org/10.1007/s10336-009-0382-6
Thomas CD, Bulman CR, Wilson RJ (2008) Where within a geographical range do species survive best? A matter of scale. Insect Conserv Divers 1:2–8. https://doi.org/10.1111/j.1752-4598.2007.00001.x
Tobolka M, Dylewski L, Wozna JT, Zolnierowicz KM (2018) How weather conditions in non-breeding and breeding grounds affect the phenology and breeding abilities of white storks. Sci Total Environ 636:512–518. https://doi.org/10.1016/j.scitotenv.2018.04.253
Trierweiler C, Klaassen RHG, Drent RH, Exo KM, Komdeur J, Bairlein F, Koks BJ (2014) Migratory connectivity and population-specific migration routes in a long-distance migratory bird. Proc Royal Soc B 281:2013–2897. https://doi.org/10.1098/rspb.2013.2897
UNEP-WCMC (2021) Protected area profile for Africa from the World Database of Protected Areas, Feb 2021. Available at: www.protectedplanet.net
Veen T, Hjernquist MB, Van Wilgenburg SL, Hobson KA, Folmer E, Font L, Klaassen M (2014) Identifying the African wintering grounds of hybrid flycatchers using a multi-isotope (δ2H, δ13C, δ15N) assignment approach. PLoS One 9(5):e98075. https://doi.org/10.1371/journal.pone.0098075
Wakelin J, McKechnie AE, Woodborne S (2011) Stable isotope analysis of migratory connectivity in a threatened intra-African migrant, the Blue Swallow (Hirundo atrocaerulea). J Ornithol 152:171–177. https://doi.org/10.1007/s10336-010-0562-4
Walther BA (2016) A review of recent ecological changes in the Sahel, with particular reference to land-use change, plants, birds and mammals. Afr J Ecol 54:268–280. https://doi.org/10.1111/aje.12350
Weber TP, Houston AI, Ens BJ (1999) Consequences of habitat loss at migratory stopover sites: a theoretical investigation. J Avian Biol 30:416–426. https://doi.org/10.2307/3677014
Webster MS, Marra PP, Haig SM, Bensch S, Holmes T (2002) Links between worlds unraveling migratory connectivity. Trends Ecol Evol 17(2):76–83. https://doi.org/10.1016/S0169-5347(01)02380-1
Wunder MB (2007) Geographic structure and dynamics in Mountain Plover. Dissertation. Fish, Colorado State University
Zwarts L, Bijlsma RG, van der Kamp J, Wymenga E (2009) Living on the edge: wetlands and birds in a changing Sahel. Chapter 32: European Turtle Dove Streptopelia turtur. 2nd edition, KNNV Publishing, Zeist 378–389
Acknowledgements
We are thankful to all the persons and organisations who helped during fieldwork, with logistics in the field and provided feather samples: France: Marcel Rivière (Oléron), Bernard Mauvy (Auvergne), Luc Tison and Hervé Bidault (Chizé), Tony Presse, Alain Richard and Denis Koniska (Marne); Italy: Sara Riello, Elisa Mancuso, Riserva Naturale Statale Isole di Ventotene e S. Stefano and LIPU Centro di Recupero Fauna Selvatica di Roma; Malta: John J. Borg (NMNH, Heritage Malta), the Maltese Ringing Scheme and BirdLife Malta; Bulgaria: Strahil Peev. We thank Yvonne Klaar, Anja Luckner, and Karin Grassow for help with the stable isotope analyses and Steven L. van Wilgenburg for contributing code and advice on assignments to origin.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
MM, YRS, and PQ conceived and designed the study. MM, PQ, GR, PZ, DB, BM, JGC, FS, MCB, HL, SF, and CE conducted fieldwork and provided samples. MM, CCV, and PQ performed the experiments. MM, YRS, KJK, and KAH performed statistical analyses and assignments to origin. MM, YRS, and PQ analysed the data. MM, YRS, and KJK wrote the manuscript; all other authors discussed the result and provided editorial advice.
Corresponding author
Ethics declarations
Ethics approval
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Consent to participate
All authors voluntarily agree to participate in the elaboration and publication of this manuscript.
Consent for publication
All authors declare that they participated in the study and the development of the manuscript, as well as read the final version and give consent for the article to be published in EJWR.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marx, M., Schumm, Y.R., Kardynal, K.J. et al. Feather stable isotopes (δ2Hf and δ13Cf) identify the Sub-Saharan wintering grounds of turtle doves from Europe. Eur J Wildl Res 68, 21 (2022). https://doi.org/10.1007/s10344-022-01567-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-022-01567-w