Abstract
Objective
An early metabolic signature associated with the responsiveness to treatment can be useful in the better management of septic shock patients. This would help clinicians in designing personalized treatment protocols for patients showing non-responsiveness to treatment.
Methods
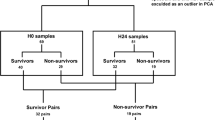
We analyzed the serum on Day 1 (n = 60), Day 3 (n = 47), and Day 5 (n = 26) of patients with septic shock under treatment using NMR-based metabolomics. Partial least square discriminant analysis (PLS-DA) was performed to generate the list of metabolites that can be identified as potential disease biomarkers having statistical significance (that is, metabolites that had a VIP score > 1, and p value < 0.05, False discovery rate (FDR) < 0.05).
Results
Common significant metabolites amongst the three time points were obtained that distinguished the patients being responsive (R) and non-responsive (NR) to treatments, namely 3 hydroxybutyrate, lactate, and phenylalanine which were lower, whereas glutamate and choline higher in patients showing responsiveness.
Discussion
The study gave these metabolic signatures identifying patients’ responsiveness to treatment. The results of the study will aid in the development of targeted therapy for ICU patients.



Similar content being viewed by others
References
Keeley A, Hine P, Nsutebu E (2017) The recognition and management of sepsis and septic shock: a guide for non-intensivists. Postgrad Med J 93:626–634. https://doi.org/10.1136/POSTGRADMEDJ-2016-134519
Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR et al (2020) Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the global burden of disease study. Lancet 395:200–211. https://doi.org/10.1016/S0140-6736(19)32989-7
Kaukonen K-M, Bailey M, Suzuki S, Pilcher D, Bellom R (2014) Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA 311:1308–1316. https://doi.org/10.1001/JAMA.2014.2637
Vincent J-L, Jones G, David S, Olariu E, Cadwell KK (2019) Frequency and mortality of septic shock in Europe and North America: a systematic review and meta-analysis. Crit Care. https://doi.org/10.1186/S13054-019-2478-6
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M et al (2016) The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315:801–810. https://doi.org/10.1001/JAMA.2016.0287
Jones AE, Trzeciak S, Kline JA (2009) The sequential organ failure assessment score for predicting outcome in patients with severe sepsis and evidence of hypoperfusion at the time of emergency department presentation. Crit Care Med 37:1649. https://doi.org/10.1097/CCM.0B013E31819DEF97
Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M et al (2017) Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 43:304–377. https://doi.org/10.1007/S00134-017-4683-6
Fleischmann C, Scherag A, Adhikari NKJ, Hartog CS et al (2016) Assessment of global incidence and mortality of hospital-treated sepsis current estimates and limitations. Am J Respir Crit Care Med 193:259–272. https://doi.org/10.1164/RCCM.201504-0781OC
Wong HR, Cvijanovich NZ, Anas N, Allen GL et al (2015) Developing a clinically feasible personalized medicine approach to pediatric septic shock. Am J Respir Crit Care Med 191:309–315. https://doi.org/10.1164/RCCM.201410-1864OC
Russell C, Rahman A, Mohammed AR (2013) Application of genomics, proteomics and metabolomics in drug discovery, development and clinic. Ther Deliv 4:395–413. https://doi.org/10.4155/TDE.13.4
Kaddurah-Daouk R, Krishnan KRR (2008) Metabolomics: a global biochemical approach to drug response and disease. Annu Rev Pharmacol Toxicol 48:653–683. https://doi.org/10.1146/ANNUREV.PHARMTOX.48.113006.094715
Balashova EE, Maslov DL, Lokhov PG (2018) A metabolomics approach to pharmacotherapy personalization. J Pers Med. https://doi.org/10.3390/JPM8030028
Siddiqui MA, Pandey S, Azim A, Sinha N, Siddiqui MH (2020) Metabolomics: an emerging potential approach to decipher critical illnesses. Biophys Chem. https://doi.org/10.1016/j.bpc.2020.106462
Mickiewicz B, Vogel HJ, Wong HR, Winston BW (2013) Metabolomics as a novel approach for early diagnosis of pediatric septic shock and its mortality. Am J Respir Crit Care Med 187:967–976. https://doi.org/10.1164/rccm.201209-1726OC
Mardegan V, Giordano G, Stocchero M, Pirillo P, Poloniato G, Donadel E et al (2021) Untargeted and targeted metabolomic profiling of preterm newborns with early-onset sepsis: a case-control study. Metabolites 11:1–15. https://doi.org/10.3390/metabo11020115
Liu Z, Triba MN, Amathieu R, Lin X, Bouchemal N, Hantz E et al (2019) Nuclear magnetic resonance-based serum metabolomic analysis reveals different disease evolution profiles between septic shock survivors and non-survivors. Crit Care 23:1–12. https://doi.org/10.1186/S13054-019-2456-Z
Jaurila H, Koivukangas V et al (2020) 1 H NMR based metabolomics in human sepsis and healthy serum. Metabolites. https://doi.org/10.3390/METABO10020070
Wang J, Sun Y, Teng S, Li K (2020) Prediction of sepsis mortality using metabolite biomarkers in the blood: a meta-analysis of death-related pathways and prospective validation. BMC Med 18:1–15. https://doi.org/10.1186/S12916-020-01546-5
Garcia-Simon M, Morales JM, Modesto-Alapont V, Gonzalez-Marrachelli V, Vento-Rehues R, Jorda-Miñana A et al (2015) Prognosis biomarkers of severe sepsis and septic shock by 1H NMR urine metabolomics in the intensive care unit. PLoS ONE 10:e0140993. https://doi.org/10.1371/journal.pone.0140993
Jaurila H, Koivukangas V, Koskela M, Gäddnäs F, Myllymaa S, Kullaa A et al (2020) 1H NMR based metabolomics in human sepsis and healthy serum. Metabolites 10:1–13. https://doi.org/10.3390/metabo10020070
Ferrario M, Cambiaghi A, Brunelli L, Giordano S, Caironi P, Guatteri L et al (2016) Mortality prediction in patients with severe septic shock: a pilot study using a target metabolomics approach. Sci Reports 6:1–11. https://doi.org/10.1038/srep20391
Cambiaghi A, Díaz R, Martinez JB, Odena A, Brunelli L, Caironi P et al (2018) An innovative approach for the integration of proteomics and metabolomics data in severe septic shock patients stratified for mortality. Sci Rep 8:6681. https://doi.org/10.1038/s41598-018-25035-1
Liu Z, Yin P, Amathieu R, Savarin P, Xu G (2016) Application of LC-MS-based metabolomics method in differentiating septic survivors from non-survivors. Anal Bioanal Chem 408:7641–7649. https://doi.org/10.1007/s00216-016-9845-9
Mickiewicz B, Tam P, Jenne CN, Leger C, Wong J, Winston BW et al (2015) Integration of metabolic and inflammatory mediator profiles as a potential prognostic approach for septic shock in the intensive care unit. Crit Care 19:11. https://doi.org/10.1186/s13054-014-0729-0
Su L, Huang Y, Zhu Y, Xia L, Wang R, Xiao K et al (2014) Discrimination of sepsis stage metabolic profiles with an LC/MS-MS-based metabolomics approach. BMJ Open Respir Res 1:e000056. https://doi.org/10.1136/bmjresp-2014-000056
Mickiewicz B, Duggan GE, Winston BW, Doig C, Kubes P, Vogel HJ (2014) Metabolic profiling of serum samples by 1H nuclear magnetic resonance spectroscopy as a potential diagnostic approach for septic shock. Crit Care Med. https://doi.org/10.1097/CCM.0000000000000142
Dalli J, Colas RA, Quintana C, Barragan- D, Hurwitz S, Levy BD et al (2018) Human sepsis eicosanoid and pro-resolving lipid mediator temporal profiles: correlations with survival and clinical outcomes. Crit Care Med 45:58–68. https://doi.org/10.1097/CCM.0000000000002014.Human
Langley RJ, Tsalik EL, van Velkinburgh JC, Glickmanm SW et al (2014) An integrated clinico-metabolomic model improves prediction of death in sepsis. Bone 23:1–7. https://doi.org/10.1126/scitranslmed.3005893.An
Hussain H, Vutipongsatorn K, Jiménez B, Antcliffe DB (2022) Patient stratification in sepsis: using metabolomics to detect clinical phenotypes, sub-phenotypes and therapeutic response. Metabolites 12(5):376. https://doi.org/10.3390/metabo12050376
Cambiaghi A, Pinto BB, Brunelli L, Falcetta F, Aletti F, Bendjelid K, Pastorelli R, Ferrario M (2017) Characterization of a metabolomic profile associated with responsiveness to therapy in the acute phase of septic shock. Sci Rep 7(1):9748. https://doi.org/10.1038/s41598-017-09619-x
Stoessel D, Stellmann J-P, Willing A, Behrens B, Rosenkranz SC, Hodecker SC, Stürner KH, Reinhardt S, Fleischer S, Deuschle C, Maetzler W, Berg D, Heesen C, Walther D, Schauer N, Friese MA, Pless O (2018) Metabolomic profiles for primary progressive multiple sclerosis stratification and disease course monitoring. Front Hum Neurosci 12:226. https://doi.org/10.3389/fnhum.2018.00226
Westerhuis JA, Hoefsloot HCJ, Smit S, Vis DJ, Smilde AK, van Velzen EJJ, van Duijnhoven JPM, van Dorsten FA (2008) Assessment of PLSDA cross validation. Metabolomics. https://doi.org/10.1007/s11306-007-0099-6
Colquhoun D (2014) An investigation of the false discovery rate and the misinterpretation of p values. R Soc opensci 1:140216. https://doi.org/10.1098/rsos.140216
Ruokonen E, Takala J, Kari A, Alhava E (1991) Septic shock and multiple organ failure. Crit Care Med 19:1146–1151. https://doi.org/10.1097/00003246-199109000-00009
Ferreira FL, Bota DP, Bross A, Mélot C, Vincent J-L (2001) Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 286:1754–1758. https://doi.org/10.1001/JAMA.286.14.1754
Levy MM, Macias WL, Vincent J-L, Russell JA, Silva E, Trzaskoma B, Williams MD (2005) Early changes in organ function predict eventual survival in severe sepsis. Crit Care Med 33:2194–2201. https://doi.org/10.1097/01.CCM.0000182798.39709.84
Maciel AT, Noritomi DT, Park M (2010) Metabolic acidosis in sepsis. Endocr Metab Immune Disord Drug Targets 10:252–257. https://doi.org/10.2174/187153010791936900
Garcia-Alvarez M, Marik P, Bellomo R (2014) Sepsis-associated hyperlactatemia. Crit Care 18:1–11. https://doi.org/10.1186/S13054-014-0503-3
Lee SM, An WS (2016) New clinical criteria for septic shock: serum lactate level as new emerging vital sign. J Thorac Dis 8:1388. https://doi.org/10.21037/JTD.2016.05.55
Davids MR, Segal AS, Brunengraber H, Halperin ML (2004) An unusual cause for ketoacidosis. QJM 97:365–376. https://doi.org/10.1093/QJMED/HCH064
Hahn PY, Wang P, Tait SM, Ba ZF, Reich SS, Chaudry IH (1995) Sustained elevation in circulating catecholamine levels during polymicrobial sepsis. Shock 4:269–273. https://doi.org/10.1097/00024382-199510000-00007
Chao Wu, Wang X, Wenkui Yu, Tian F et al (2015) Hypermetabolism in the initial phase of intensive care is related to a poor outcome in severe sepsis patients. Ann Nutr Metab 66:188–195. https://doi.org/10.1159/000430848
Frankenfield DC, Omert LA, Badellino MM et al (1994) Correlation between measured energy expenditure and clinically obtained variables in trauma and sepsis patients. JPEN J Parenter Enteral Nutr 18:398–403. https://doi.org/10.1177/0148607194018005398
Grey NJ, Karl I, Kipnis DM (1975) Physiologic mechanisms in the development of starvation ketosis in man. Diabetes 24:10–16. https://doi.org/10.2337/DIAB.24.1.10
Geisler S, Gostner JM, Becker K, Ueberall F, Fuchs D (2013) Immune activation and inflammation increase the plasma phenylalanine-to-tyrosine ratio. Pteridines 24:27–31. https://doi.org/10.1515/PTERID-2013-0001
Jeremias IC, Victorino VJ et al (2016) The role of acetylcholine in the inflammatory response in animals surviving sepsis induced by cecal ligation and puncture. Mol Neurobiol 53:6635–6643. https://doi.org/10.1007/S12035-015-9538-Y
Poeze M, Luiking YC, Breedveld P (2008) Decreased plasma glutamate in early phases of septic shock with acute liver dysfunction is an independent predictor of survival. Clin Nutr 27:523–530. https://doi.org/10.1016/J.CLNU.2008.04.006
Zhang Y, Yu W, Han D, Meng J, Wang H, Cao G (2019) L–lysine ameliorates sepsis-induced acute lung injury in a lipopolysaccharide-induced mouse model. Biomed Pharmacother 118:109307. https://doi.org/10.1016/J.BIOPHA.2019.109307
Luiking YC, Deutz NEP (2007) Biomarkers of arginine and lysine excess. J Nutr 137:1662S-1668S. https://doi.org/10.1093/JN/137.6.1662S
Merz TM, Pereira AJ, Schürch R, Schefold JC, Jakob SM, Takala J et al (2017) Mitochondrial function of immune cells in septic shock: a prospective observational cohort study. PLoS ONE 12:e0178946. https://doi.org/10.1371/JOURNAL.PONE.0178946
Kitamura H, Yamauchi A, Sugiura T et al (1998) Inhibition of myo-inositol transport causes acute renal failure with selective medullary injury in the rat. Kidney Int 53:146–153. https://doi.org/10.1046/J.1523-1755.1998.00747.X
Izquierdo-Garcia JL, Nin N, Cardinal-Fernandez P, Rojas Y, De Paula M, Granados R et al (2019) Identification of novel metabolomic biomarkers in an experimental model of septic acute kidney injury. Am J Physiol Ren Physiol 316:54–62. https://doi.org/10.1152/ajprenal.00315.2018
Hasselgren PO, Fischer JE (1998) Sepsis: stimulation of energy-dependent protein breakdown resulting in protein loss in skeletal muscle. World J Surg 22:203–208. https://doi.org/10.1007/S002689900370
Acknowledgements
Authors acknowledge Centre of Biomedical Research for providing the infrastructure and resources and Centre of Scientific and Industrial Research(CSIR), INDIA, for research fellowship.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
NS, AA and SP conceptualized and designed the methodology of the study. NS, AA and SP investigated the study. NS and AA provided the resources and supervised the study. NS and AA administered the project. NS provided financial support for the project. Formal analysis using software, data curation and validation was performed by SP. Patient sample and clinical data collection by MAS and SP. Visualization and original draft preparation by SP. Final draft review and editing by SP, MAS, AA and NS. All authors approved the final submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors have disclosed that they do not have any potential conflicts of interest.
Ethical approval
The study was approved by the Institutional Ethical Committee of Sanjay Gandhi Post Graduate Institute of Medical Sciences with IEC code:2018-170-PhD-107 and approval code: PEG/BI/37/2019 and date of approval 28.01.19.
Informed consent statement
Informed consent was obtained from all subjects involved in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pandey, S., Siddiqui, M.A., Azim, A. et al. Metabolic fingerprint of patients showing responsiveness to treatment of septic shock in intensive care unit. Magn Reson Mater Phy 36, 659–669 (2023). https://doi.org/10.1007/s10334-022-01049-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-022-01049-9