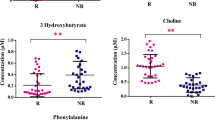
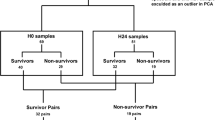
Abstract
Septic shock is the most severe form of sepsis, which is still one of the leading causes of death in the intensive care unit (ICU). Even though early prognosis and diagnosis are known to be indispensable for reaching an optimistic outcome, pathogenic complexities and the lack of specific treatment make it difficult to predict the outcome individually. In the present study, serum samples from surviving and non-surviving septic shock patients were drawn before clinical intervention at admission. Metabolic profiles of all the samples were analyzed by liquid chromatography-mass spectrometry (LC-MS)-based metabolomics. One thousand four hundred nineteen peaks in positive mode and 1878 peaks in negative mode were retained with their relative standard deviation (RSD) below 30 %, in which 187 metabolites were initially identified by retention time and database in the light of the exact molecular mass. Differences between samples from the survivors and the non-survivors were investigated using multivariate and univariate analysis. Finally, 43 significantly varied metabolites were found in the comparison between survivors and non-survivors. Concretely, metabolites in the tricarboxylic acid (TCA) cycle, amino acids, and several energy metabolism-related metabolites were up-regulated in the non-survivors, whereas those in the urea cycle and fatty acids were generally down-regulated. Metabolites such as lysine, alanine, and methionine did not present significant changes in the comparison. Six metabolites were further defined as primary discriminators differentiating the survivors from the non-survivors at the early stage of septic shock. Our findings reveal that LC-MS-based metabolomics is a useful tool for studying septic shock.

ᅟ




Similar content being viewed by others
References
Cao Z, Yende S, Kellum JA, Angus DC, Robinson RA. Proteomics reveals age-related differences in the host immune response to sepsis. J Proteome Res. 2014;13(2):422–32.
Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock—a review of laboratory models and a prospal. J Surg Res. 1980;29(2):189–201.
Messing B, Peitracohen S, Debure A, Beliah M, Bernier JJ. Antibiotic-lock technique—a new approach to optimal therapy for catheter-related sepsis in home - parenteral nutrition patients. Jpen-Parenter Enter. 1988;12(2):185–9.
Vincent F-L. New therapies in sepsis. Chest. 1997;112(6):330S–8.
Cao Z, Robinson RA. The role of proteomics in understanding biological mechanisms of sepsis. Proteomics Clin Appl. 2014;8(1–2):35–52.
Zhao X, Chen YX, Li CS. Predictive value of the complement system for sepsis-induced disseminated intravascular coagulation in septic patients in emergency department. J Crit Care. 2015;30(2):290–5.
Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–93.
Christaki E, Giamarellos-Bourboulis EJ. The beginning of personalized medicine in sepsis: small steps to a bright future. Clin Genet. 2014;86(1):56–61.
Shane AL, Stoll BJ. Neonatal sepsis: progress towards improved outcomes. J Infect. 2014;68 Suppl 1:S24–32.
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. New Engl J Med. 2001;345(19):1368–77.
Lam SW, Bauer SR, Guzman JA. Septic shock: the initial moments and beyond. Cleve Clin J Med. 2013;80(3):175–84.
Antti H, Fahlgren A, Nasstrom E, Kouremenos K, Sunden-Cullberg J, Guo Y, et al. Metabolic profiling for detection of Staphylococcus aureus infection and antibiotic resistance. PLoS One. 2013;8(2):e56971.
Mickiewicz B, Duggan GE, Winston BW, Doig C, Kubes P, Vogel HJ, et al. Metabolic profiling of serum samples by 1H nuclear magnetic resonance spectroscopy as a potential diagnostic approach for septic shock. Crit Care Med. 2014;42(5):1140–9.
Cho SY, Choi JH. Biomarkers of sepsis. Infect Chemother. 2014;46(1):1–12.
Henriquez-Camacho C, Losa J. Biomarkers for sepsis. Biomed Res Int. 2014;2014:547818.
Hinkelbein J, Kalenka A, Schubert C, Peterka A, Feldmann Jr RE. Proteome and metabolome alterations in heart and liver indicate compromised energy production during sepsis. Protein Peptide Lett. 2010;17(1):18–31.
Lindon JC, Holmes E, Nicholson JK. Metabonomics techniques and applications to pharmaceutical research & development. Pharm Res. 2006;23(6):1075–88.
Lin ZY, Xu PB, Yan SK, Meng HB, Yang GJ, Dai WX, et al. A metabonomic approach to early prognostic evaluation of experimental sepsis by (1)H NMR and pattern recognition. NMR Biomed. 2009;22(6):601–8.
Izquierdo-Garcia JL, Nin N, Ruiz-Cabello J, Rojas Y, de Paula M, Lopez-Cuenca S, et al. A metabolomic approach for diagnosis of experimental sepsis. Intensive Care Med. 2011;37(12):2023–32.
Xu PB, Lin ZY, Meng HB, Yan SK, Yang Y, Liu XR, et al. A metabonomic approach to early prognostic evaluation of experimental sepsis. J Infect. 2008;56(6):474–81.
Fanos V, Caboni P, Corsello G, Stronati M, Gazzolo D, Noto A, et al. Urinary 1H-NMR and GC-MS metabolomics predicts early and late onset neonatal sepsis. Early Hum Dev. 2014;90:S78–83.
Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36(1):296–327.
Minne L, Abu-Hanna A, de Jonge E. Evaluation of SOFA-based models for predicting mortality in the ICU: a systematic review. Crit Care. 2008;12(6):R161.
Vincent JL, Moreno R, Takala J, Willatts S, DeMendonca A, Bruining H, et al. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. Intens Care Med. 1996;22(7):707–10.
van Beek JH, de Moor MH, de Geus EJ, Lubke GH, Vink JM, Willemsen G, et al. The genetic architecture of liver enzyme levels: GGT, ALT and AST. Behav Genet. 2013;43(4):329–39.
Chen J, Zhao X, Fritsche J, Yin P, Schmitt-Kopplin P, Wang W, et al. Practical approach for the identification and isomer elucidation of biomarkers detected in a metabonomic study for the discovery of individuals at risk for diabetes by integrating the chromatographic and mass spectrometric information. Anal Chem. 2008;80(4):1280–9.
Gasparetto A, Corbucci GG, Candiani A, Gohil K, Edwards RHT. Effect of tissue hypoxia and septic shock on human skeletal muscle mitochondria. Lancet. 1983;322(8365–8366):1386.
Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360(9328):219–23.
Whelan SP, Carchman EH, Kautza B, Nassour I, Mollen K, Escobar D, et al. Polymicrobial sepsis is associated with decreased hepatic oxidative phosphorylation and an altered metabolic profile. J Surg Res. 2014;186(1):297–303.
Hasselgren PO, Fischer JE. Sepsis: stimulation of energy-dependent protein breakdown resulting in protein loss in skeletal muscle. World J Surg. 1998;22:203–8.
Bremer J. Carnitine—metabolism and functions. Physiol Rev. 1983;63(4):1420–80.
Flanagan JL, Simmons PA, Vehige J, Willcox MD, Garrett Q. Role of carnitine in disease. Nutr Metab (Lond). 2010;7:30.
Trivedi V, Bavishi C, Jean R. Impact of obesity on sepsis mortality: a systematic review. J Crit Care. 2015;30(3):518–24.
Forni LG, McKinnon W, Lord GA, Treacher DF, Peron JM, Hilton PJ. Circulating anions usually associated with the Krebs cycle in patients with metabolic acidosis. Crit Care. 2005;9(5):R591–5.
Kim SC, Pierro A, Zamparelli M, Spitz L, Eaton S. Fatty acid oxidation in neonatal hepatocytes: effects of sepsis and glutamine. Nutrition. 2002;18(4):298–300.
Steelman SM, Johnson P, Jackson A, Schulze J, Chowdhary BP. Serum metabolomics identifies citrulline as a predictor of adverse outcomes in an equine model of gut-derived sepsis. Physiol Genomics. 2014;46(10):339–47.
Liu XR, Zheng XF, Ji SZ, Lv YH, Zheng DY, Xia ZF, et al. Metabolomic analysis of thermally injured and/or septic rats. Burns. 2010;36(7):992–8.
Luo L, Schomaker S, Houle C, Aubrecht J, Colangelo JL. Evaluation of serum bile acid profiles as biomarkers of liver injury in rodents. Toxicol Sci. 2014;137(1):12–25.
Mickiewicz B, Vogel HJ, Wong HR, Winston BW. Metabolomics as a novel approach for early diagnosis of pediatric septic shock and its mortality. Am J Respir Crit Care Med. 2013;187(9):967–76.
Zhang W, Zhou L, Yin P, Wang J, Lu X, Wang X, et al. A weighted relative difference accumulation algorithm for dynamic metabolomics data: long-term elevated bile acids are risk factors for hepatocellular carcinoma. Sci Rep. 2015;5:8984.
Zhou L, Ding L, Yin P, Lu X, Wang X, Niu J, et al. Serum metabolic profiling study of hepatocellular carcinoma infected with hepatitis B or hepatitis C virus by using liquid chromatography-mass spectrometry. J Proteome Res. 2012;11(11):5433–42.
Chen T, Xie G, Wang X, Fan J, Qiu Y, Zheng X, et al. Serum and urine metabolite profiling reveals potential biomarkers of human hepatocellular carcinoma. Mol Cell Proteomics. 2011;10(7):M110.004945.
Seymour CW, Yende S, Scott MJ, Pribis J, Mohney RP, Bell LN, et al. Metabolomics in pneumonia and sepsis: an analysis of the GenIMS cohort study. Intensive Care Med. 2013;39(8):1423–34.
Patel SS, Molnar MZ, Tayek JA, Ix JH, Noori N, Benner D, et al. Serum creatinine as a marker of muscle mass in chronic kidney disease: results of a cross-sectional study and review of literature. J Cachex Sarcopenia Muscle. 2013;4(1):19–29.
Yoshifuji A, Wakino S, Irie J, Tajima T, Hasegawa K, Kanda T, et al. Gut lactobacillus protects against the progression of renal damage by modulating the gut environment in rats. Nephrol Dial Transplant Off Publ Eur Dial Transplant Assoc Eur Ren Assoc. 2016;31(3):401–12.
Ridgway ND, Yao Z, Vance DE. Phosphatidylethanolamine levels and regulation of phosphatidylethanolamine N-methyltransferase. J Biol Chem. 1989;264(2):1203–7.
Mayers JR, Wu C, Clish CB, Kraft P, Torrence ME, Fiske BP, et al. Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nat Med. 2014;20(10):1193–8.
Xu L, Huang D, Hu Q, Wu J, Wang Y, Feng J. Betaine alleviates hepatic lipid accumulation via enhancing hepatic lipid export and fatty acid oxidation in rats fed with a high-fat diet. Br J Nutr. 2015;113(12):1835–43.
Acknowledgments
We sincerely thank to the support of the University of Paris-XIII and the help from the Jean Verdier Hospital. The study has been supported by the key foundation (No. 21435006) from the National Natural Science Foundation of China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The Institutional Review Board of Jean Verdier University Hospital approved the protocol and the French Research Delegation Office accepted the creation of a dedicated bio-collection for the patients included in the protocol. The CNIL (National Informatics and Liberty Commission) also approved the creation of both bio-collection and database. Depending of the mental status of the patient, the written consent was obtained directly from the patient at inclusion or from the Person of Confidence designed by the patient (pre-emptively) or by the family relatives. In case of survival, a secondary written consent from the patient was systematically seek.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 428 kb)
Rights and permissions
About this article
Cite this article
Liu, Z., Yin, P., Amathieu, R. et al. Application of LC-MS-based metabolomics method in differentiating septic survivors from non-survivors. Anal Bioanal Chem 408, 7641–7649 (2016). https://doi.org/10.1007/s00216-016-9845-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9845-9