Abstract
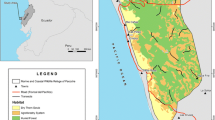
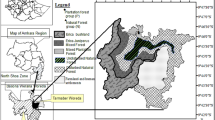
Historical records of Ateles chamek (black-faced black spider monkey) suggest that the species range extends further south of the known species distribution, within an ecotonal region between the Amazonia, Cerrado and Pantanal biomes in Brazil. Ecotones are zones of habitat transition with high species richness that remain undersampled as conservationists often prioritize biodiversity hotspots. Thus, distribution ranges may be inaccurately measured when species occur in ecotonal zones. We report the first precise records of A. chamek in 24 new localities surveyed in the ecotonal zone of the Upper Paraguay River Basin, and we present subgroup encounter rates in the 11 largest patches (>70 ha) along 207 km of the line transects surveyed. The new records represent an expansion of the distribution of A. chamek approximately 200 km to the south, increasing the known extent of its occurrence by 10.8%. Local tributaries may not be barriers for spider monkeys, which are able to swim and cross slow-moving rivers. However, the dry forests of the Cerrado and the flooded areas of the Pantanal, formed by grassland and scarce trees, may be habitat barriers for A. chamek. The populations living in this ecotonal zone are relatively abundant (1.1–6.67 subgroup sightings/10 km) compared to the heavily hunted continuous forests of northern Amazonia. Furthermore, these values are similar to those for other Ateles spp. inhabiting forests with low or no hunting pressure. We highlight the need for specific conservation action to protect the spider monkeys living in these landscapes, which are threatened by agriculture expansion.

Similar content being viewed by others
References
Alho CJR, Fischer E, Oliveira-Pissini LF, Santos CF (2011) Bat-species richness in the Pantanal floodplain and its surrounding uplands. Braz J Biol 71:311–320
Amaral DL, Fonzar BC (1982) Levantamento de recursos naturais. Projeto RADAMBRASIL. Resource document. MME, Cuiabá, Rio de Janeiro, p SD 21
Aquino R, Bodmer RE (2006) Distribución y abundancia de Ateles belzebuth E. Geoffroy y Ateles chamek Humboldt (Cebidae: primates) en la reserva nacional Pacaya Samiria. Perú. Rev Peru Biol 13:103–106
Aquino R, Alvarez J, Mulanovich A (2005) Diversidad y estado de conservación de primates en las Sierras de Contamana, Amazonía Peruana. Rev Peru Biol 12:427–434
Aquino R, Cornejo FM, Pezo E, Heymann EW (2012) Distribution and abundance of white-fronted spider monkeys, Ateles belzebuth (Atelidae), and threats to their survival in Peruvian Amazonia. Folia Primatol 84:1–10
Aquino R, Cornejo FM, Heymann EW (2013) Primate abundance and habitat preferences on the lower Urubanda and Tambo rivers in central eastern Peruvian Amazonia. Primates 54:377–383
Ayres JM, Clutton-Brock TH (1992) River boundaries and species range size in Amazonian primates. Am Nat 140:531–537
Bicknell J, Peres CA (2010) Vertebrate population responses to reduced-impact logging in a Neotropical forest. For Ecol Manage 259:2267–2275
Cáceres NC, Hannibal W, Freitas DR, Silva EL, Roman C, Casella J (2010) Mammal occurrence and roadkill in two adjacent ecoregions (Atlantic Forest and Cerrado) in south-western Brazil. Zoologia 27:709–717
Campbell CJ, Aureli F, Chapman CA, Ramos-Fernández G, Matthews K, Russo SE, Suarez S, Vick L (2005) Terrestrial behavior of Ateles spp. Int J Primatol 26:1039–1051
Cant JGH (1990) Fedding ecology of spider monkeys (Ateles geoffroyi) at Tikal Guatemala. Hum Evol 5:269–281
Castrillon SKI, Silva CJD, Fernandez JRC, Ikeda AK (2011) Assessment of tree diversity on the islands of the Paraguay River in the Cáceres region, Pantanal Brazil. Acta Bot Bras 25:672–684
Chapman C (1987) Flexibility in diets of three species of Costa Rican primates. Folia Primatol 29:90–105
Chaves O, Stoner KE (2010) Habilidad para cuzar ríos en Ateles geoffroyi y Alouatta pigra en el sur de México: Un reporte preliminar. Rev Chil Hist Nat 83:435–442
Collins AC, Dubach JM (2000) Biogeographic and ecological forces responsible for speciation in Ateles. Int J Primatol 21:421–444
Cristóbal-Azkarate J, Urbani B, Asensio N (2015) Interactions of howler monkeys with other vertebrates: a review. In: Kowalewski MM, Garber PA, Cortés-Ortiz L, Urban B, Youlatos D (eds) howler monkeys. Springer, New York, pp 141–164
da Silva CJ, Sousa KNS, Ikeda-Castrillon SK, Lopes CRAS, da Silva Nunes JR, Carniello MA, Mariotti PR, Lazaro WL, Morini A, Zago BW, Façanha CL (2015) Biodiversity and its drivers and pressures of change in the wetlands of the Upper Paraguay-Guaporé Ecotone, Mato Grosso (Brazil). Land Use Policy 47:163–178
Eiten G (1993) Vegetação do Cerrado. In: Novaes Pinto M (ed) Cerrado: caracterização, ocupação e perspectivas. Universidade de Brasília, Brasília, pp 17–73
Endo W, Peres CA, Salas E, Mori S, Sanchez-Vega JL, Shepard GH, Pacheco V, Yu DW (2010) Game vertebrate densities in hunted and nonhunted forest sites in Manu National Park, Peru. Biotropica 42:251–261
Galetti M, Sazima I (2006) Impact of feral dogs in an urban Atlantic forest fragment in southeastern Brazil. Nat Conserv 4:146–151
Godoi MN, Morante Filho JC, Módena ÉS, Faxina C, Tizianel FAT, Bocchese R, Pivatto MAC, Nunes AP, Posso SR (2014) Birds of Upper Paraná river Basin in the State of Mato Grosso do Sul, Brazil. Rev Bras Ornitol 21:176–204
Goldani A, Carvalho GS, Bicca-Marques JC (2006) Distribution patterns of Neotropical primates (Platyrrhini) based on parsimony analysis of endemicity. Braz J Biol 66:61–74
Gusmão AC, Crispim MA, Ferronato ML, Junior JDSES (2014) Primatas da Reserva Particular do Patrimônio Natural Água Boa, Cacoal, Rondônia, Brasil. Neotrop Primates 21:207–209
IUCN (2012) Guidelines for application of IUCN Red List criteria at regional and national levels: version 4.0
Iwanaga S, Ferrari SF (2002) Geographic distribution and abundance of woolly (Lagothrix cana) and spider monkeys (Ateles chamek) in southwesten Brazilian Amazonia. Am J Primatol 56:57–64
Kark S, Van Rensburg BJ (2006) Ecotones: marginal or central areas of transition? Isr J Ecol Evol 52:29–53
Konstant WR, Rylands AB (2013) Species accounts of Ateles. In: Mittermeier RA, Rylands AB, Wilson DE (eds) Handbook of the mammals of the world, vol 3, primates. Lynx, Barcelona, pp 536–542
Lapola DM, Martinelli LA, Peres CA, Ometto CA, Ferreira JP, Aguiar AP, Bustamante MCM, Cardoso MF, Costa MH, Joly CA, Leite CC, Moutinho P, Sampaio G, Strassburg BNB, Vieira ICG (2014) Pervasive transition of the Brazilian land-use system. Nat Clim Change 4:27–35
Marsh C, Link A, King-Bailey G, Donati G (2016) Effects of fragment and vegetation structure on the population abundance of Ateles hybridus, Alouatta seniculus and Cebus albifrons in Magdalena Valley, Colombia. Folia Primatol 87:17–30
Marshall AR, Lovett JC, White PC (2008) Selection of line-transect methods for estimating the density of group-living animals: lessons from the primates. Am J Primatol 70:452–462
Martinelli LA, Filoso S (2008) Expansion of sugarcane ethanol production in Brazil: environmental and social challenges. Ecol Appl 18:885–898
Milton K (1993) Diet and social organization of a free-ranging spider monkey population: the development of species-typical behavior in the absence of adults. In: Pereira ME, Fairbanks LA (eds) Juvenile primates: life history, development and behavior. Chicago University Press, Chicago, pp 173–181
Miranda-Ribeiro A (1914) História natural. Zoologia, Comissão de Linhas telegraphicas estratégicas de Matto-Grosso ao Amazonas
Mittermeier RA (1987) Effects of hunting on rain forest primates. In: Marsh CW, Mittermeier RA, Liss AR (eds.) Primate conservation in the tropical rain forest. New York, Oryx, pp 109–146
Mittermeier RA, Kinzey WG, Mast RB (1989) Neotropical primate conservation. J Hum Evol 18:597–610
Nepstad D, McGrath D, Stickler C, Alencar A, Azevedo A, Swette B, Bezerra T, DiGiano M, Shimada J, Motta RS, Armijo E, Castello L, Brando P, Hansen MC, McGrath-Horn M, Carvalho O, Hess L (2014) Slowing Amazon deforestation through public policy and interventions in beef and soy supply chains. Science 344:1118–1123
Norconck MA, Sussman RW, Phillips-Conroy JP (1996) Primates of Guyana shield forests. In: Norconck MA, Rosenberger AL, Garber PA (eds) Adaptive radiations of Neotropical primates. Plenum, New York, pp 69–83
Nunes AV (2014) Report of a black spider monkey (Ateles chamek) swimming in a large river in central-western Brazil. Neotrop Primates 21:204–206
Palminteri S, Powell G, Endo W, Kirkby C, Yu D, Peres CA (2011) Usefulness of species range polygons for predicting local primate occurrences in southeastern Peru. Am J Primatol 73:53–61
Peres CA (1997) Primate community structure at twenty western Amazonian flooded and unflooded forests. J Trop Ecol 13:381–405
Peres CA, Cunha A (2011) Line-transect censuses of large-bodied tropical forest vertebrates: a handbook. Wildlife Conservation Society, Brasilia
Porfirio G, Sarmento P, Xavier Filho NL, Cruz J, Fonseca C (2014) Medium to large size mammals of southern Serra do Amolar, Mato Grosso do Sul, Brazilian Pantanal. Check List 10:473–482
Rabelo RM, Silva FE, Vieira T et al (2014) Extension of the geographic range of Ateles chamek (Primates, Atelidae): evidence of river-barrier crossing by an Amazonian primate. Primates 55:167–171
Redford KH, Robinson JG (1987) The game of choice: patterns of indian and colonist hunting in the Neotropics. Am Anthropol 89:650–667
Rosin C, Swamy V (2013) Variable density responses of primate communities to hunting pressure in a western Amazonian River Basin. Neotrop Primates 20:25–31
Rylands AB, Mittermeier RA (2013) Family Atelidae ((howlers, spider and woolly monkeys and muriquis). In: Mittermeier RA, Rylands AB, Wilson DE (eds) Handbook of the mammals of the world. Lynx, Barcelona, pp 484–549
Santos-Filho M, Peres CA, da Silva DJ, Sanaiotti TM (2012) Habitat patch and matrix effects on small-mammal persistence in Amazonian forest fragments. Biodivers Conserv 21:1127–1147
Smith TB, Wayne RK, Girman DJ, Bruford MW (1997) A role for ecotones in generating rainforest biodiversity. Science 276:1855–1857
Smith TB, Kark S, Schneider CJ, Wayne RK, Moritz C (2001) Biodiversity hotspots and beyond: the need for preserving environmental transitions. Trees 16:431
Symington MM (1988) Demography, ranging patterns and activity budgets of black spider monkeys (Ateles paniscus chamek) in the Manu National Park, Peru. Am J Primatol 15:45–67
Tomas WM, Cáceres NC, Nunes AP, Fischer E, Mourão G, Campos Z (2010) Mammals in the Pantanal wetland, Brazil. In: Junk WJ, da Silva CJ, Nunes C, Wantzen KM (eds) The Pantanal: ecology, biodiversity and sustainable management of a large Neotropical seasonal wetland. Pensoft, Sofia-, Moscow, pp 563–595
Valadão RM (2012) Birds of the Estação Ecológica Serra das Araras, Mato Grosso, Brazil. Biota Neotrop 12:263–281
Van Der Hammen T (1982) Paleoecology of tropical South America. In: Prance GT (ed) Biological diversification in the tropics. Columbia University Press, New York, pp 60–65
Van Roosmalen MGM (1980) Habitat preferences, diet, feeding, strategy and social organization of the black spider monkey (Ateles panisius paniscus L.) in Surinam. (Doctoral dissertation). Agricultural University of Wageningen., Leersum. Available from: Wageningen UR Library Catalogue, Wageningen, Netherlands, n826
Van Roosmalen MGM, Klein LL (1988) The spider monkeys, genus Ateles. In: Mittermeier RA, Coimbra-Filho AB, Fonseca GAB (eds) Ecology and behavior of Neotropical primates, vol 2. WWF, Washington, DC, pp 455–537
Wallace RB (2005) Seasonal variations in diet and foraging behavior of Ateles chamek in a southern Amazonian tropical forest. Int J Primatol 26:1053–1075
Wallace RB, Mittermeier RA, Cornejo F, Boubli JP (2008) Ateles chamek. In: IUCN Red List of Threatened Species version 2013.2. http://www.iucnredlist.org. Downloaded 19 December 2016
Weghorst JA (2007) High population density of black-handed spider monkeys Ateles geoffroyi in Costa Rican lowland wet forest. Primates 48:108–116
Acknowledgements
We would like to thank all the landowners for permission to work within their landholdings. We thank Giovanna C. Olinto for proofreading the manuscript. We also acknowledge Dr. Phyllis C. Lee and Dr. Collen M. Schaffner for their valuable comments and suggestions regards this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by the Cooordination for the Improvement of Higher Education Personnel (A. C. G, H. W. V. L. B. and C. S. S. B.) and financial support from FAPEMAT (Edital—UNIVERSAL, DOUTOR/FAPEMAT no. 005-2012).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
In adhering to the legal requirements of Brazil, no animals were captured or harmed during this research. The interviews were conducted in accordance with Brazilian legislation (law no. 5197 of 3 January 1967).
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
dos Santos-Filho, M., Bernardo, C.S.S., Van der Laan Barbosa, H.W. et al. A new distribution range of Ateles chamek (Humboldt 1812) in an ecotone of three biomes in the Paraguay River Basin. Primates 58, 441–448 (2017). https://doi.org/10.1007/s10329-017-0601-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10329-017-0601-3