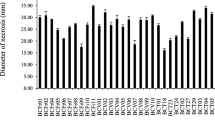
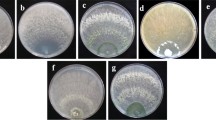
Abstract
Native Trichoderma spp. were isolated from agricultural fields in several regions of Ecuador. These isolates were characterized via morphological observation as well as molecular phylogenetic analysis based on DNA sequences of the rDNA internal transcribed spacer region, elongation factor-1α gene and RNA polymerase subunit II gene. Fifteen native Trichoderma spp. were identified as T. harzianum, T. asperellum, T. virens and T. reesei. Some of these strains showed strong antagonistic activities against several important pathogens in Ecuador, such as Fusarium oxysporum f. sp. cubense (Panama disease) and Mycosphaerella fijiensis (black Sigatoka) on banana, as well as Moniliophthora roreri (frosty pod rot) and Moniliophthora perniciosa (witches’ broom disease) on cacao. The isolates also showed inhibitory effects on in vitro colony growth tests against Japanese isolates of Fusarium oxysporum f. sp. lycopersici, Alternaria alternata and Rosellinia necatrix. The native Trichoderma strains characterized here are potential biocontrol agents against important pathogens of banana and cacao in Ecuador.



Similar content being viewed by others
References
Abdullah F, Ilias GNM, Nelson M (2007) Hyperparasitic mechanisms employed by the fungal biocontrol agent in a Trichoderma–Ganoderma interaction. In: Exploring life as a catalyst for technological advancement. Proc 9th Symp Malaysian Soc Appl Biol, Universiti Sains Malaysia. Malaysian Soc Appl Biol, Kuala Lumpur, pp 107–130
Adebesin AA, Odebode CA, Ayodele AM (2009) Control of postharvest rots of banana fruits by conidia and culture filtrates of Trichoderma asperellum. J Plant Prot Res 3:302–308
Aime MC, Phillips-Mora W (2005) The causal agents of witches’ broom and frosty pod rot of cacao (chocolate, Theobroma cacao) form a new lineage of Marasmiaceae. Mycologia 97:1012–1022
Bowers JH, Bailey BA, Hebbar PK, Sanogo S, Lumsden RD (2001) The impact of plant diseases on world chocolate production. Plant Health Progress. doi:10.1094/PHP-2001-0709-01-RV (online)
Carsolio C, Gutiérrez A, Jiménez B, Van Montagu M, Herrera-Estrella A (1994) Characterization of ech-42, a Trichoderma harzianum endochitinase gene expressed during mycoparasitism. Proc Natl Acad Sci USA 91:10903–10907
Chaverri P, Castlebury LA, Samuels G, Geiser DM (2003) Multilocus phylogenetic structure within the Trichoderma harzianum/Hypocrea lixii complex. Mol Phylogenet Evol 27:302–313
Druzhinina IS, Kopchinskiy AG, Komoń M, Bissett J, Szakacs G, Kubicek CP (2005) An oligonucleotide barcode for species identification in Trichoderma and Hypocrea. Fungal Genet Biol 42:813–828
Druzhinina IS, Kubicek CP, Komoń-Zelazowska M, Mulaw TB, Bissett J (2010) The Trichoderma harzianum demon: complex speciation history resulting in coexistence of hypothetical biological species, recent agamospecies and numerous relict lineages. BMC Evol Biol 10:94
Elad Y, Chet I, Henis Y (1981) Biological control of Rhizoctonia solani in strawberry fields by Trichoderma harzianum. Plant Soil 60:245–254
Ezziyyani M, Pérez Sánchez C, Sid Ahmed A, Requema ME, Candela ME (2004) Trichoderma harzianum como biofungicida para el biocontrol de Phytophthora capsici en plantas de pimiento (Capsicum annuum L) (in Spanish). Anal Biol 26:35–45
Garber RC, Yoder OC (1983) Isolation of DNA from filamentous fungi and separation into nuclear, mitochondrial, ribosomal, and plasmid components. Anal Biochem 135:416–422
Garcia Simões ML, Tauk-Tornisielo SM, Niella GR, Tapia Tapia DM (2012) Evaluation of Trichoderma spp. for the biocontrol of Moniliophthora perniciosa subgroup 1441. J Biol Life Sci 3:18–36
Hanada RE, Pomella AWV, Soberanis W, Loguercio LL, Pereira JO (2009) Biocontrol potential of Trichoderma martiale against the black-pod disease (Phytophthora palmivora) of cacao. Biol Control 50:143–149
Harjono K, Widyastuti SM (2001) Optimization of endochitinase production from mycoparasitic fungi Trichoderma reesei. Indon J Plant Protect 7:55–58
Harman GE, Chet I, Baker R (1981) Factors affecting Trichoderma hamatum applied to seeds as biocontrol agent. Phytopathology 71:569–572
Harman GE, Howell CR, Viterbo A, Chet I, Lorito M (2004) Trichoderma species-opportunistic, avirulent plant symbionts. Nat Rev Microbiol 2:43–56
Hermosa MR, Grondona I, Iturriaga EA, Díaz-Mínguez JM, Castro C, Monte E, García-Acha I (2000) Molecular characterization and identification of biocontrol isolates of Trichoderma spp. Appl Environ Microbiol 66:1890–1898
Hjeljord LG, Tronsmo A (1998) Trichoderma and Gliocladium in biological control: an overview. In: Harman GE, Kubicek CP (eds) Trichoderma and Gliocladium, vol 2: Enzymes, biological control and commercial applications. Taylor and Francis, London; CRC Press, Boca Raton, pp 129–151
Hjeljord LG, Tronsmo A (2003) Effect of germination initiation on competitive capacity of Trichoderma atroviride P1 conidia. Phytopathology 93:1593–1598
Hoyos-Carvajal L, Duque G, Orduz PS (2008) Antagonismo in vitro de Trichoderma spp. sobre aislamientos de Sclerotinia spp. y Rhizoctonia spp. (in Spanish). Rev Colomb Cienc Hortic 2:76–86
Jeger MJ, Jeffries P, Elad Y, Xu XM (2009) A generic theoretical model for biological control of foliar plant diseases. J Theor Biol 256:201–214
Karthikenyan M, Radhika K, Mathiyazhagan S, Bhaskaran R, Samiyappan R, Velazhahan R (2006) Induction of phenolics and defense-related enzymes in coconut (Cocos nucifera L.) roots treated with biocontrol agents. Braz J Plant Physiol 18:367–377
Kim CS, Park MS, Kim SC, Maekawa N, Yu SH (2012) Identification of Trichoderma, a competitor of shiitake mushroom (Lentinula edodes), and competition between Lentinula edodes and Trichoderma species in Korea. Plant Pathol J 28:137–148
Kopchinskiy A, Komoń M, Kubicek CP, Druzhinina IS (2005) TrichoBLAST: a multilocus database of phylogenetic markers for Trichoderma and Hypocrea identifications. Mycol Res 109:658–660
Krauss U, Hidalgo E, Bateman R, Adonijah V, Arroyo C, García J, Crozier J, Brown NA, Martijn ten Hoopen G, Holmes KA (2010) Improving the formulation and timing of application of endophytic biocontrol and chemical agents against frosty pod rot (Moniliophthora roreri) in cocoa (Theobroma cacao). Biol Control 54:230–240
Kullnig-Gradinger CM, Krupica T, Woo SL, Mach RL, Rey M, Benítez T, Lorito M, Kubicek CP (2001) Confusion abounds over identities of Trichoderma biocontrol isolates. Mycol Res 105:769–772
Kullnig-Gradinger CM, Szakacs G, Kubicek CP (2002) Phylogeny and evolution of the genus Trichoderma: a multigene approach. Mycol Res 106:757–767
Lieckfeldt E, Samuels GJ, Nirenberg HI, Petrini O (1999) A morphological and molecular perspective of Trichoderma viride: Is it one or two species? Appl Environ Microbiol 65:2418–2428
Liu YJ, Hall BD (2004) Body plan evolution of ascomycetes, as inferred from an RNA polymerase II phylogeny. Proc Natl Acad Sci USA 101:4507–4512
Lo CT (1998) General mechanisms of action of microbial biocontrol agents. Plant Pathol Bull 7:155–166
Matheny PB (2005) Improving phylogenetic inference of mushrooms with RPB1 and RPB2 nucleotide sequences (Inocybe, Agaricales). Mol Phylogenet Evol 35:1–20
Mukherjee M, Horwitz BA, Sherkhane PD, Hadar R, Mukherjee PK (2006) A secondary metabolite biosynthesis cluster in Trichoderma virens: evidence from analysis of genes underexpressed in a mutant defective in morphogenesis and antibiotic production. Curr Genet 50:193–202
Nelson EB (1991) Handbook of applied mycology. In: Arora DK, Rai B, Mukerji KG, Knudsen GR (eds) Current limits to biological control of fungal phytopathogens Vol 1: Soil and plants. Marcel Dekker, New York, p 800
Pérez L, Batlle A, Chacón J, Montenegro V (2009) Eficacia de Trichoderma harzianum A34 en el biocontrol de Fusarium oxysporum f. sp. cubense, agente causal de la marchitez de por fusarium o mal de Panamá de los bananos en Cuba (in spanish). Fitosanidad 4:259–263
Ploetz RC (2006) Fusarium wilt of banana is caused by several pathogens referred to as Fusarium oxysporum f. sp. cubense. Phytopathology 96:653–656
Qualhato TF, Cardoso Lopes FA, Stecca Steindorff A, Silva Brandão R, Amorim Jesuino RS, Ulhoa CJ (2013) Mycoparasitism studies of Trichoderma species against three phytopathogenic fungi: evaluation of antagonism and hydrolytic enzyme production. Biotechnol Lett 35:1461–1468
Rosado IV, Rey M, Codón AC, Govantes J, Moreno-Mateos MA, Benítez T (2007) QID74 Cell wall protein of Trichoderma harzianum is envolved in cell protection and adherence to hydrophobic surfaces. Fungal Genet Biol 44:950–964
Rubio MB, Hermosa R, Reino JL, Collado IG, Monte E (2008) Thctf1 transcription factor of Trichoderma harzianum is envolved in 6-pentyl-2H-pyran-2-one production and antifungal activity. Fungal Genet Biol 46:17–27
Samuels GJ (2006) Trichoderma: systematics, the sexual state, and ecology. Proceedings of the Annual Meeting of the American Phytopathological Society. APS, Rehoboth Beach, pp 195–206
Samuels GJ, Lieckfeldt E, Nirenberg HI (1999) Trichoderma asperellum, a new species with warted conidia, and redescription of Trichoderma viride. Sydowia 51:71–88
Samuels GJ, Ismaiel A, Bon MC, De Respinis S, Petrini O (2010) Trichoderma asperellum sensu lato consist of two cryptic species. Mycologia 102:944–966
Samuels GJ, Chaverri P, Farr DF, McCray EB (2014) Trichoderma online, systematic mycology and microbiology laboratory, ARS, USDA. http://nt.ars-grin.gov/taxadescriptions/keys/TrichodermaIndex.cfm
Scherm B, Schmoll M, Balmas V, Kubicek CP, Migheli Q (2009) Identification of potential marker genes for Trichoderma harzianum strains with high antagonistic potential against Rhizoctonia solani by a rapid subtraction hybridization approach. Curr Genet 55:81–91
Seidl V, Gamauf C, Druzhinina I, Seiboth B, Hartl L, Kubicek C (2008) The Hypocrea jecorina (Trichoderma reesei) hypercellulolytic mutant RUT C30 lacks a 85 kb (29 gene-encoding) region of the wild-type genome. BMC Gen 9:327
Sharma K, Mishra AK, Misra RS (2009) Morphological, biochemical and molecular characterization of Trichoderma harzianum isolated for their efficiency as biological control agents. J Phytopathol 157:51–56
Stover RH (1962) Fusarial wilt (panama disease) of bananas and other Musa species. CMI, Kew
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Watanabe S, Kumakura K, Kato H, Iyozumi H, Togawa M, Nagayama K (2005) Identification of Trichoderma SKT-1, a biological control agent against seedborne pathogens of rice. J Gen Plant Pathol 71:351–356
White TJ, Bruns T, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, New York, pp 315–322
Woo SL, Lorito M (2007) Exploiting the interactions between fungal antagonists, pathogens and the plant for biocontrol. In: Vurro M, Gressel J (eds) Novel biotechnologies for biocontrol agent enhancement and management. Springer, Netherlands, pp 107–130
Wood GAR, Lass RA (eds) (2001) Tropical agricultural series, Cocoa, 4th edn. Blackwell Science, Oxford
Zhang Cl, Druzhinina IS, Kubicek CP, Xu T (2005) Trichoderma biodiversity in China: evidence for a north to south distribution of species in East Asia. FEMS Microbiol Lett 251:251–257
Acknowledgments
We thank T. Arie, T. Yasuda and D. G. Gilchrist for providing the fungal strains. This work was supported by the Global COE Program “Advanced Utilization of Fungus/Mushroom Resources for Sustainable Society in Harmony with Nature,” MEXT, Japan. We thank the National Secretary of Higher Education, Science, Technology and Education of Ecuador and the Biotechnology Research Center of Ecuador, Higher Polytechnic College of the Littoral CIBE-ESPOL for supporting this research.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10327_2015_587_MOESM1_ESM.tiff
Supplementary material 1 (TIFF 920 kb) Supplementary Fig. 1. Dual cultures to test antagonism of Trichoderma harzianum strains (T1, T3, T15, T19, T20 and T36) against pathogenic fungi and single cultures of each fungal pathogen after 10 days at 25 °C. A 5-mm-diameter mycelial disk of the respective fungi was placed on plates with the pathogen on lower side, Trichoderma spp. on upper side. (Foc) Fusarium oxysporum f. sp. cubense (Fo-01), (Mf) Mycosphaerella fijiensis (Ec-01), (Mr) Moniliophthora roreri (Cp-01), (Mp) Mo. perniciosa (MrEO-1), (Fol) F. oxysporum f. sp. lycopersici (Chz1-A), (Aa) Alternaria alternata tomato pathotype (As-27), (Rn) Rosellinia necatrix (ES-0601).
10327_2015_587_MOESM2_ESM.tiff
Supplementary material 2 (TIFF 1114 kb) Supplementary Fig. 2. Dual cultures to test antagonism of Trichoderma harzianum strains (T2, T4, T5, T9, T10, T13 and T18) against pathogenic fungi and single cultures of each fungal pathogen after 10 days at 25°C. A 5-mm-diameter mycelial disk of the respective fungi was placed on plates with the pathogen on lower side, Trichoderma spp. on upper side. Pathogens: (Foc) Fusarium oxysporum f. sp. cubense (Fo-01), (Mf) Mycosphaerella fijiensis (Ec-01), (Mr) Moniliophthora roreri (Cp-01), (Mp) Mo. perniciosa (MrEO-1), (Fol) F. oxysporum f. sp. lycopersici (Chz1-A), (Aa) Alternaria alternata tomato pathotype (As-27), (Rn) Rosellinia necatrix (ES-0601).
10327_2015_587_MOESM3_ESM.tiff
Supplementary material 3 (TIFF 983 kb) Supplementary Fig. 3. Dual cultures to test antagonism of Trichoderma reesei strain (T29) and T. virens strain (T43) against pathogenic fungi and single cultures of each fungal pathogen after 10 days at 25°C. A 5-mm-diameter mycelial disk of the respective fungi was placed on plates with the pathogen on lower side, Trichoderma spp. on upper side. Pathogens: (Foc) Fusarium oxysporum f. sp. cubense (Fo-01), (Mf) Mycosphaerella fijiensis (Ec-01), (Mr) Moniliophthora roreri (Cp-01), (Mp) Mo. perniciosa (MrEO-1), (Fol) F. oxysporum f. sp. lycopersici (Chz1-A), (Aa) Alternaria alternata tomato pathotype (As-27), (Rn) Rosellinia necatrix (ES-0601).
Rights and permissions
About this article
Cite this article
Galarza, L., Akagi, Y., Takao, K. et al. Characterization of Trichoderma species isolated in Ecuador and their antagonistic activities against phytopathogenic fungi from Ecuador and Japan. J Gen Plant Pathol 81, 201–210 (2015). https://doi.org/10.1007/s10327-015-0587-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10327-015-0587-x