Abstract
The current energy crisis, depletion of fossil fuels, and global climate change have made it imperative to find alternative sources of energy that are both economically sustainable and environmentally friendly. Here we review various pathways for converting biomass into bioenergy and biochar and their applications in producing electricity, biodiesel, and biohydrogen. Biomass can be converted into biofuels using different methods, including biochemical and thermochemical conversion methods. Determining which approach is best relies on the type of biomass involved, the desired final product, and whether or not it is economically sustainable. Biochemical conversion methods are currently the most widely used for producing biofuels from biomass, accounting for approximately 80% of all biofuels produced worldwide. Ethanol and biodiesel are the most prevalent biofuels produced via biochemical conversion processes. Thermochemical conversion is less used than biochemical conversion, accounting for approximately 20% of biofuels produced worldwide. Bio-oil and syngas, commonly manufactured from wood chips, agricultural waste, and municipal solid waste, are the major biofuels produced by thermochemical conversion. Biofuels produced from biomass have the potential to displace up to 27% of the world's transportation fuel by 2050, which could result in a reduction in greenhouse gas emissions by up to 3.7 billion metric tons per year. Biochar from biomass can yield high biodiesel, ranging from 32.8% to 97.75%, and can also serve as an anode, cathode, and catalyst in microbial fuel cells with a maximum power density of 4346 mW/m2. Biochar also plays a role in catalytic methane decomposition and dry methane reforming, with hydrogen conversion rates ranging from 13.4% to 95.7%. Biochar can also increase hydrogen yield by up to 220.3%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
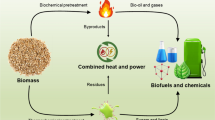
Biomass is a renewable and sustainable energy source that has received considerable attention due to the need to reduce greenhouse gas emissions and reliance on fossil fuels (Osman et al. 2022a, 2023). Biomass conversion to bioenergy and products with added value, such as biochar, can occur via several pathways. Thermochemical conversion processes involve heating biomass at high temperatures without or in the presence of a limited amount of oxygen, producing bioenergy and biochar. Typically, techniques such as direct combustion, torrefaction, hydrothermal liquefaction, pyrolysis, and gasification are employed in this process (Osman et al. 2021). In contrast, biological conversion pathways utilize microorganisms to decompose biomass to produce biogas and biofuels, including biodiesel, ethanol, and bio-hydrogen (Farghali et al. 2022). This method typically employs fermentation and anaerobic digestion to break down the complex organic molecules present in biomass into simpler components that can be utilized as fuel, as shown in Fig. 1.
Biomass conversion pathways into biofuels and other high-value applications. Different forms of biomass, including woody, herbaceous, waste, and aquatic types, undergo thermochemical or biological conversion processes to produce biofuels, for instance, biogas in the form of gas, liquid biofuels, or solid form such as biochar. The produced biochar is considered a value-added product. It can be utilized in diverse energy applications, including electricity generation via microbial fuel cells, biohydrogen, and biodiesel production
In this review, we examine various pathways of biomass conversion to bioenergy and biochar, as well as their applications in electricity production, biodiesel production, and biohydrogen production. Our main emphasis is optimizing biomass pathways and exploring the use of biochar in renewable energy. Furthermore, we evaluate biomass energy's environmental impact and sustainability and compare its efficiency and proportion to other renewable energy sources.
Pathways of biomass conversion
Two major ways for biomass conversion into energy include thermochemical and biological transformations. Usually, physicochemical treatments, such as particle size reduction, drying, densification, and solvent fractionalization, are applied as pretreatments before other conversion methods. Thermochemical conversion technologies include torrefaction, pyrolysis, gasification, and hydrothermal processing. Biological conversions include alcohol fermentation (bioethanol) and anaerobic digestion (biomethane). Biomass thermochemical modification products, such as biochar, could enhance the biological conversion of biomass in anaerobic digestion and ethanol fermentation.
Thermochemical processes
Direct combustion
It is one of the most traditional and widely used methods for converting biomass into energy, as shown in Fig. 2. The process involves burning biomass in a boiler or furnace, which generates heat that can be used to produce steam or electricity (Kanwal et al. 2021). Nevertheless, biomass combustion differs depending on the chemical characterization of the raw material because of its chemical composition and heating value (Briones-Hidrovo et al. 2021). Drying, pyrolysis, and reduction are the first three steps in biomass combustion, followed by the combustion of volatile gases and solid char (Aliyu et al. 2021).
Direct combustion process. It comprises five major subsystems: waste feeding, heat exchange, incineration, waste heat boiler, and flue gas cleaning systems. The high-temperature flue gas created by biomass combustion heated the feed water via heat transfer, producing superheated steam that powered the steam turbine to provide more power. The flue gas might then be vented to the atmosphere through the stack after being cleaned by the flue gas cleaning system
The two most popular boiler designs are fluidized-bed combustion chambers and grate heating systems, which offer high fuel flexibility (Ye et al. 2020). The most fundamental part of combustion technology is a furnace in which biomass is burnt. The choice and design of any biomass combustion system are primarily influenced by the properties of the fuel, environmental restrictions, costs, and plant scale (Fang et al. 2019). Direct biomass combustion generates heat that may be used to directly power steam turbines that turn generators to produce electricity or high-pressure steam that can be used to heat buildings, businesses, and districts (Aliyu et al. 2021).
Direct combustion is better suited for biomass feedstock with low moisture content. Nevertheless, algal biomass has greater moisture content and produces exhaust smoke and fumes; direct combustion is not appropriate as the primary method for producing energy (Ayub et al. 2022). One study suggested that the algal feedstock must be dried to a moisture content of 20% to be suitable for direct combustion (Mathimani et al. 2019). Microalgae biomass co-combustion with coal, on the other hand, may create sustainable and cost-effective energy and reduce greenhouse gas emissions (Choi et al. 2019). Direct combustion of wet municipal solid waste was carried out to assess the energy recovery potential of direct burning. It was found that municipal solid waste combustion plants may consume at least 67% of the collected municipal solid waste and add an extra 3% of electrical energy to the national energy grid (Gutierrez-Gomez et al. 2021).
Direct combustion has several advantages over other biomass conversion technologies. It is a relatively simple and mature technology, with many existing installations that can be easily retrofitted to use biomass instead of fossil fuels. Additionally, it can be used with a wide range of feedstocks, including wood, agricultural residues, and municipal solid waste, which can help reduce waste disposal costs and promote sustainability. However, direct combustion also has some challenges. The process can produce emissions such as particulate matter and nitrogen oxides, contributing to air pollution and having negative health impacts. The efficiency of the process can also vary depending on the biomass feedstock’s quality and the combustion system's design (Sivabalan et al. 2021).
Torrefaction
Biomass torrefaction can take place in either dry or moist circumstances. Biomass can be torrefied in non-oxidative (inert) or oxidative atmospheres at temperatures ranging from 200 to 300 °C in dry torrefaction (He et al. 2021). In non-oxidative torrefaction, nitrogen and carbon dioxide have been used as carrier gases. When thermally treated biomass materials, nitrogen is the most often employed carrier gas to sweep them; in oxidative torrefaction, pretreating biomass using gases with changing oxygen concentrations as carrier gases has been investigated. Because of the presence of oxygen and the exothermic processes that occur during thermal degradation, oxidative torrefaction has a faster response rate than non-oxidative torrefaction (Chen et al. 2021a).
Torrefaction has several advantages over other biomass conversion technologies, as shown in Fig. 3 and Table 1. Torrefied biomass has a higher energy density and lower moisture content, making it easier to handle, transport, and store than raw biomass (Abdulyekeen et al. 2021). Torrefaction processes are designed to enhance the characteristics of the treated biomass, such as grindability, a decreased capacity to absorb moisture, lower molecular compositional oxygen-to-carbon and hydrogen-to-carbon ratios, and better heating values (Rago et al. 2020). The process also removes impurities such as volatile organic compounds, which reduces emissions during combustion and increases the efficiency of the process. Torrefaction can also be used with various feedstocks, including wood chips, agricultural residues, and municipal solid waste. This can help reduce waste disposal costs and promote sustainability (Akbari et al. 2021; Isemin et al. 2021). The torrefaction process also improves the quality of biomass-derived solid fuels (Barskov et al. 2019).
Torrification process setup. The reactor is fed raw biomass at the intake, and the reactor drum spins on a horizontal axis. The biomass is heated indirectly by recycled torrefaction gas flowing through the revolving drum, and the screw drives the biomass ahead through the twisted screw of the reactor. These reactors maintain process continuity and are small in size. Finally, the torrefied hydrochar is released at the reactor outlet
Wet torrefaction is more commonly used for animal manures, sewage slugs, and municipal garbage because wet samples are heated in a pressure chamber while submerged in water, eliminating the requirement for pre-drying at temperatures ranging from 180 to 260 °C (Xue et al. 2021). The solid char product derived from wet torrefaction, known as hydrochar, differs significantly from that derived from dry torrefaction. Furthermore, hydrochar has a better heating value than biochar and contains fewer heavy metals, alkaline earth, and alkali metals than biochar most of the time (Alkharabsheh et al. 2021).
Recently, a study examined the biomass of torrefaction of cotton stalks in an ammonia medium. The synthesis of pyridines was aided by ammonia torrefaction, whereas the quantity of oxygen-containing species was reduced (Zhao et al. 2022a). Torrefaction was recently examined as a pretreatment alternative for synthesizing biohydrogen from corn stover. Torrefaction enhanced reducing sugar yield and biohydrogen generation substantially compared to untreated maize stover (Chen et al. 2021b). Paulownia wood was examined to study the effect of particle size on torrefaction characteristics at various torrefaction temperatures and periods. The composition of torrefied gas products was considerably influenced by torrefaction temperature, but particle size and torrefaction duration had a minimal impact (He et al. 2023).
Several kinetic models have been investigated, ranging from simple single-step to sophisticated multi-step models with underlying reaction processes. Two-step models are the most popular option because of their reputation for simplicity and the quality of the outcomes they produce. The model successfully estimates the solid mass loss and volatile development during torrefaction (Chen et al. 2021a; Feng et al. 2022).
However, torrefaction also has some challenges. The process can require significant energy input to achieve the desired temperature and residence time, which can increase the cost of production. Additionally, the process can result in the loss of some valuable components, such as sugars and amino acids, which can limit the potential use of the torrefied biomass as a feedstock for certain products. Despite these challenges, torrefaction is a promising technology that has the potential to play a significant role in meeting our energy needs while reducing our dependence on fossil fuels and mitigating climate change. With further research and development, it may become a key component of a more sustainable and renewable energy future.
Hydrothermal liquefaction
Biomass may be transformed into high-value products like biofuels, chemicals, and fertilizers using hydrothermal liquefaction, as shown in Fig. 4 and Table 2. In the presence of a catalyst, wet biomass is heated at high temperatures ranging from 200 to 374 °C and pressures ranging from 15 to 220 bar, causing the material to break down into a liquid known as bio-oil (Beims et al. 2020). The main advantage of the hydrothermal liquefaction technique is that it may employ biomass with a high moisture level, such as algae, municipal sludge, lignocellulose, or an organic component of municipal solid waste, without requiring a drying procedure (Jatoi et al. 2022). Biomass is converted into bio-oi, the primary product, solid residue, aqueous phase, and gas phase product under hydrothermal liquefaction conditions (Xu and Li 2021).
Hydrothermal liquefaction process. The process involves pyrolyzing and liquefying organic biomass during specific inorganic components precipitate as solids. A continuous-flow reactor system is used to separate the crude product from the byproduct water and recover mineral and gas byproducts for analysis. The system comprises a high-pressure feeding subsystem, consisting of a feed tank and a dual-piston pump, along with a preheater, reactor, and separator that are heated by a circulating oil heater. The mineral separation step is done via a high-pressure vessel, and the liquid products are directly separated by gravity to aqueous and bio-crude phases. The solids separator vessel is designed as a quiescent vessel where solids settle at the bottom and liquids pass overhead through a tubular filter element
The reaction temperature, retention duration, heating rate, biomass type, solid content, solvent type, catalyst type, and catalyst concentration all affect product yield and quality in hydrothermal liquefaction processes (Lachos-Perez et al. 2022). Several attempts have been made to improve bio-crude oil output and quality using an acidic/alkaline catalyst in biomass liquefaction. The addition of an alkaline catalyst was studied by using ethanol/water co-solvents or pure water to liquefy pinewood sawdust with or without using sodium carbonate or sodium hydroxide as a catalyst. Both catalysts improved the liquefaction of pinewood sawdust in clean water by boosting biomass breakdown and the generation of bio-crude oil. When sodium carbonate was used as the catalyst, it had a more substantial catalytic impact on oil yield than sodium hydroxide (Hu et al. 2020).
Another significant aspect of the process was the liquefaction solvent using a batch reactor for hydrothermal liquefaction of corn straw. The maximum yield of bio-oil was obtained from a mixed solvent of methanol and water, while methanol, ethyl alcohol, and isopropanol resulted in a lower yield of bio-oil. The effect of adding homogeneous catalysts (sodium carbonate and potassium carbonate) and heterogeneous catalysts (zeolite catalysts and NKC-11) was also investigated. NKC-11 obtained the maximum bio-oil yield, and heterogeneous catalysts produced more bio-oil than homogeneous catalysts (Zhang et al. 2020a).
Using the same reaction conditions, the hydrothermal liquefaction procedure was conducted on four different kinds of agricultural straw: rice straw, peanut straw, soybean straw, and maize straw. Of the four crop straws, soybean straw provided the greatest bio-oil production of 15.8 wt% (Tian et al. 2020). Even plastic waste stained with food waste can be used as feedstocks for large-scale reactors (Shimahata et al. 2022).
The bio-oil produced by hydrothermal liquefaction has several advantages over other biomass conversion technologies. It is similar in composition to crude oil and can be refined into transportation fuels, such as diesel and jet fuel. It can also be used as a feedstock for producing chemicals and other high-value products, such as bio-based plastics and fertilizers (Wang et al. 2020a). However, hydrothermal liquefaction also has some challenges. The process can be energy-intensive and require significant water, making it difficult to scale up in regions with water scarcity. The quality of the bio-oil can also be variable and may require additional processing to remove impurities and stabilize the product (Low et al. 2021).
Pyrolysis
It involves heating biomass to high temperatures without oxygen, which causes the material to break into a mixture of gases, liquids, and solids, as shown in Fig. 5 and Table 3. It requires temperatures between 350 °C and 700 °C (Osman et al. 2023, 2022b; Yogalakshmi et al. 2022). When biomass is pyrolyzed, it creates solid, liquid, and gas products. The gases produced by pyrolysis are typically rich in carbon monoxide, hydrogen, and methane and can be used as fuel or a feedstock for producing chemicals. The liquids produced are known as bio-oil, while the solids are known as biochar (Babinszki et al. 2021; Farghali et al. 2022). Dehydration, depolymerization, isomerization, decarboxylation, aromatization, and char production are all simultaneous chemical processes that take place during the breakdown and reaction process known as the pyrolysis of biomass (Osman et al. 2023; Ravindran et al. 2021).
Method of the pyrolysis process. The pyrolysis process involves several components, including a reactor, biomass receiving system, biomass drying and grinding equipment, product collection unit, and storage facility. The resulting char byproduct can be used for combustion or separated and exported. Efficient and rapid char separation is crucial, and this is often achieved through the use of cyclones. The liquid collection is carried out via indirect heat exchange to minimize evaporation. The collection of aerosols, which significantly contribute to liquid yield, is achieved through demisters, with electrostatic precipitation or coalescence methods being common options
The pyrolysis process can be categorized based on the heating rate. Slow pyrolysis happens over a longer period with very slow heating rates (higher than 10 °C/min), in which biochar is the main product (Bhoi et al. 2020). The fundamental purpose of intermediate pyrolysis is to produce a mixture of slow and quick pyrolysis products. Slow pyrolysis produces high solid yields while producing low liquid yields, and quick pyrolysis produces high liquid yields while producing low solid yields. Temperatures between 300 and 600 °C and heating rates between 0.1 and 10 °C/min are often employed for intermediate pyrolysis (Wang et al. 2022).
Fast pyrolysis produces large bio-oil yields using high-speed heating rates with short residence periods (Li et al. 2021a). Flash pyrolysis uses even greater heating rates, with the primary products being gases and bio-oil. With the primary purpose of preventing the re-polymerization of degraded products, flash pyrolysis is becoming increasingly popular as a viable method for synthesizing liquid fuels from biomass utilizing very high temperatures and shorter reaction times (Ighalo et al. 2022). Several biomass pyrolysis methods have recently arisen, such as microwave-assisted pyrolysis being widely researched due to its benefits over conventional pyrolysis (Wang et al. 2020c).
Microwave radiation enters the biomass particle and is converted into heat inside the particle for microwave heating. Heat continually builds inside the biomass particle and is transported externally because of the heat loss effect of the particle surface (Fahmy et al. 2020). The fuel, thermal, and surface characteristics of biochar produced from four feedstocks (canola straw, sawdust, wheat straw, and manure pellet) were investigated using microwave pyrolysis at three different temperatures. The yield of biochar obtained from lignocellulosic feedstocks increased with temperature as the concentration of elemental oxygen decreased (Kwak et al. 2019; Nzediegwu et al. 2021).
Several properties of the pyrolysis products can be directly affected by pyrolysis operation conditions; however, some are more significant than others. Temperature is considered a crucial and significant process parameter during biomass pyrolysis. Because of its extensive impact on other pyrolysis parameters, controlling the temperature profile is essential at temperatures above 150 °C biochar is formed, with the release of pyrolysis gases and a reduction in char yields (Yogalakshmi et al. 2022). Biomass degradation occurs due to destroying the cellulose and hemicellulose components within the biomass materials. At 300–500 °C, bio-oil yields rose while biochar yields declined dramatically (Amenaghawon et al. 2021).
Pyrolysis has several advantages over other biomass conversion technologies. It can produce a cleaner and more efficient energy source than combustion, with fewer pollutants like nitrogen oxides and sulfur dioxide emissions. The process can also be used with various feedstocks, including wood chips, agricultural waste, and municipal solid waste. However, pyrolysis also has some challenges. The process can be energy-intensive and requires careful control of temperature and other conditions to achieve optimal results. The quality of the bio-oil can also be variable and may require additional processing to remove impurities and stabilize the product. Additionally, the process can require significant capital investment and ongoing maintenance, making it difficult for small-scale operations to be economical (Saravanan et al. 2022b; Yek et al. 2022).
Gasification
Unlike combustion, which burns biomass to produce heat and power, gasification converts biomass into a gas called syngas through controlled oxidation, as shown in Fig. 6 and Table 4 (Mishra & Upadhyay, 2021). The process involves heating biomass to high temperatures (700–1000 °C) in the presence of a limited amount of oxygen or steam, which causes the biomass to break down into a mixture of gases, including hydrogen, carbon monoxide, and methane (Sajid et al. 2022). The syngas can then be used as a fuel to generate electricity and heat or as a feedstock for producing chemicals and other products (Hu et al. 2021).
Gasification procedure. The gasification unit comprises various components, including a fuel tank, fuel delivery system, gasification generator with a fixed-bed reactor, high-temperature filter, gas cooling device, scrubber, fan, and gas cooling device an afterburning chamber for the generated gas. In the gasification process, the air is utilized as the gasification medium. The gas produced is discharged from the top of the reactor and proceeds to a high-temperature filter to eliminate solid particles. Subsequently, the gas is cooled down using a water cooler and scrubber, eliminating some of the tars and moisture
Early in the eighteenth century, the gasification process created tar due to its capacity to reduce volume and produce energy. Now, the main challenge in gasification is the development of tar, which is challenging to remove and reduces hydrogen output (Li et al. 2022). The composition and purity of the generated gas are maintained by the feed composition, technology employed, process type, and gasifier design (Tezer et al. 2022). Biomass gasification, which aims to convert biomass into synthesis gas (including carbon monoxide and hydrogen), requires using a gasifying medium such as air, steam, oxygen, or supercritical water (Hanchate et al. 2021). Because of its ease of availability and low cost, the air is the most utilized gasifying medium. However, when steam was used as the gasification medium, the resultant gas had a more excellent hydrogen/carbon ratio (Cao et al. 2020). Oxygen gasification generates the greatest heating value syngas as carbon conversion improves, tar content reduces, and the amount of hydrogen and carbon monoxide in the generated gas rises (AlNouss et al. 2020).
Another critical factor in the gasification process is the gasifier. The demand for the products, the amount of moisture, and the availability of fuel affect the size and type of gasifier (Zhang et al. 2019d). Because of their simple design and ease of operation, fixed-bed gasifiers are the most often used commercial gasifiers (Ren et al. 2019). Fixed-bed gasifiers can be further divided into groups based on how the gasifying agent and biomass interact (Ren et al. 2020). The biomass is loaded from the upper section of the reactor, and the gasifying agent is delivered from the lower part of the reactor in the updraft gasifier (Calì et al. 2020). While in downdraft gasifiers, biomass is loaded from the top part of the reactor, the gasifying agent is delivered to the system via channels established in the center of the reactor, and synthesis gas is extracted from the bottom portion of the reactor (Barontini et al. 2021). In a cross-flow gasifier, biomass is introduced into the top section of the reactor while air is supplied from the side. The resulting gases are extracted from the opposite side of the unit at the same level (Siwal et al. 2020).
Fluidized-bed gasifiers are another gasifier that provides a well-mixing for gas and solids and homogeneous temperature distribution (Kwong et al. 2021). Based on the medium's velocity, fluidized-bed gasifiers are categorized into bubbling and circulating (Agu et al. 2019). Large-scale businesses can benefit from fluidized-bed gasifiers since they are more efficient than fixed-bed gasifiers and can operate at larger powers.
The gasification temperature primarily impacts product quality, tar formation, reactor demand, and capital expenditures. The gasification and partial pressure of the gasifying agents significantly influence gasification performance and the quality of the resulting gas. When selecting a gasifier, bed material plays an important role. It can be inert or catalytic, depending on the process conditions and demand (Ren et al. 2019). Inert materials include silica and alkaline metal oxides. Nevertheless, nickel- and potassium-based catalysts in bed can influence operating parameters such as pressure, temperature, and product gas composition (Mishra and Upadhyay 2021).
Recently, co-gasification attracted much attention because the hydrogen-to-carbon monoxide ratio may change with excellent process efficiency. By modifying the feedstock mixing ratio, the heating value of syngas will be enhanced. Also, the significant compositional variance of feedstock may be controlled to obtain the dependable process design and desired product configuration (Hameed et al. 2021). There is the influence of blending ratios of sawdust with municipal solid waste on syngas quality. Because pine sawdust has a lower carbon content than municipal solid waste, increasing the pine sawdust proportion lowered the carbon monoxide fraction while increasing the hydrogen fraction in the product stream (Cao et al. 2019).
Aspen Plus has been used in designing a gasification system to handle municipal solid waste and switchgrass to produce high-quality syngas with minimal tar content. The results revealed that adding steam to the gasification agent might increase the hydrogen concentration and gas generation. The composition of the generated syngas rarely changed, while the percentage of municipal solid waste in the mixture of municipal solid waste and switchgrass was altered (Hu et al. 2021). Gasification has several advantages over other biomass conversion technologies. It can produce a cleaner and more efficient energy source than combustion, with fewer pollutants like nitrogen oxides and sulfur dioxide emissions. The process can also be used with various feedstocks, including wood chips, agricultural waste, and municipal solid waste. This can help reduce waste disposal costs and promote sustainability (Sajid et al. 2022).
However, gasification also has some challenges. The process can be complex and requires careful temperature control and other conditions to achieve optimal results. The gas produced can also be low in energy content and may require additional processing to purify and separate different components. Gasification can also require significant capital investment and ongoing maintenance, making it difficult for small-scale operations to be economically viable (Megía et al. 2021).
Biological conversion
Fermentation
Regarding biofuels, fermentation is popular for converting biomass into bio-hydrogen and other biofuels using microorganisms like yeast and bacteria, as shown in Fig. 7 and Table 5 (Bhatia et al. 2021). During the process, complex sugars in the feedstock are broken down into simpler molecules through the action of enzymes produced by microorganisms. The biofuel is then separated from the remaining liquid and purified for use. Fermentation may be divided into dark and photo fermentation (Saravanan et al. 2022a).
Fermentation process of many subprocesses, such as milling saccharification, fermentation, distillation, rectification, and dehydration. Starches are extracted from grains and converted to sugars for fermentation. A certain amount of fresh water, enzymes, and yeast extract is designed to be additionally supplemented to the fermentation reactor. After distillation, products are processed into biogas, electricity, heat, and fertilizer. Dried grains from distilleries can be kept and/or transferred great distances to livestock feedlots. These byproducts generate additional revenue for biohydrogen production. The process also produces carbon dioxide, which is converted into green electricity
In dark fermentation, many fermentative bacteria can produce bio-hydrogen using a diverse spectrum of organic biomass or wastes as a substrate. The hydrogenase enzyme will aid hydrogen production during fermentation (Mishra et al. 2019). Under anaerobic circumstances, hydrogen protons will function as electron acceptors, neutralizing the electrons created during the oxidation of organic substrate and producing hydrogen. As oxygen is reduced during aerobic respiration, the only byproduct is water (Osman et al. 2021). The highest hydrogen output in dark fermentation depends on substrate type, inoculum, and process factors such as pH, temperature, and others. Many reactor configurations have been used in dark fermentation, such as continuously stirred tank reactors, membrane reactors, packed-bed reactors, and anaerobic fluidized-bed reactors (Brindhadevi et al. 2021). One of the simplest reactors is the continuous stirred tank reactor, which has several benefits, including a straightforward design and improved control over operational parameters (Sillero et al. 2022). In comparison, membrane bioreactors were utilized in dark fermentation because of their numerous advantages, including reducing microbial washout, producing high-quality effluent, and improving solid removal (Sim et al. 2021).
Temperature is one of the critical factors influencing bio-hydrogen generation in both pure and mixed cultures. It not only affects the generating bacteria but also affects substrate degradation and enzyme activity. The dark fermentation of rice straw showed much better results at 55 °C than 37 °C fermentation due to greater glucose and formate breakdown (Chen et al. 2022a). Most studies found optimal pH settings between 5.5 and 6.5 resulted in maximum enzyme efficiency and microbial growth. As the operational pH fluctuates above or below the ideal limits, there is a significant decrease in biohydrogen production (Ziara et al. 2019).
Hydraulic retention time is the average amount of time a soluble component spends within the reactor. Hydraulic retention time is one of the key factors influencing how well hydrogen is used by bacteria that consume it and convert organic materials into biohydrogen and organic acids (Ferreira et al. 2020). A recent study found that shortening hydraulic retention time from 8 to 3 h increased biohydrogen yield and production rate (Gorgec and Karapinar 2019). The presence of nanoparticles during dark fermentation increased biohydrogen production due to numerous characteristics such as more specialized nature, larger surface area-to-volume ratio, and stronger catalytic activity. Ferric oxide/date seed-activated carbon nanocomposites were investigated using Enterobacter aerogenes by dark fermentation, and 238.7 mL H2/g was produced, which is almost three times greater than the one without carbon nanoparticles (Rambabu et al. 2021).
Fermentation has many advantages over other biomass conversion technologies. It is a low-cost and low-energy process that can produce high-quality biofuels from a wide range of feedstocks. It is also a natural and environmentally friendly process that produces fewer greenhouse gas emissions than fossil fuels and can help reduce dependence on foreign oil (Baeyens et al. 2020). However, fermentation does have some downsides. It can be a fickle process that is highly sensitive to changes in temperature, pH, and other environmental factors, which can affect the efficiency of the process. The yield of biofuels produced by fermentation can also be limited by the availability of sugars and the efficiency of the microorganisms used in the process. Additionally, fermentation can produce large amounts of waste, such as spent yeast and other byproducts, which can be challenging to dispose of (Mishra et al. 2019).
Anaerobic digestion
It is a natural process that converts biomass into biogas through the action of microorganisms without oxygen, as shown in Fig. 8 and Table 6. It is an efficient and environmentally friendly way to convert organic waste into energy and valuable byproducts (Kumar and Samadder 2020; Lu et al. 2022). The process involves feeding organic materials into a digester, where microorganisms break down the waste and produce biogas and digestate. Biogas is a mixture of methane and carbon dioxide, which can be used to generate electricity and heat or as a transportation fuel(Farghali et al. 2022). Digestate is a nutrient-rich byproduct that can be used as a fertilizer or soil amendment (Theuerl et al. 2019). While anaerobic digestion happens in various natural settings, regulated design and technical procedures are utilized to commercially create biogas in a digester (Cai et al. 2021). Biogas mainly comprises methane and carbon dioxide with minor quantities of other gases. Even though anaerobic digestion processes have been in use for decades, they continue to be an appealing method of energy generation as an environmentally friendly alternative to fossil-based energy (Abubakar & Sciences, 2022).
Anaerobic digestion plant design. The non-biodegradable elements are separated from the organic fraction through wet mechanical pretreatment of trash utilizing a hydropulper and hydrocyclone. After that, the organic fraction is suspended to facilitate easier disintegration during the subsequent wet anaerobic digestion phase. The digester is fed automatically and semi-continuously for brief times throughout the day. High liquid levels will hinder the digester feed pump in the digestion exit sump
Generating biogas from lignocellulosic substrates presents an outstanding possibility to transform considerable biomass resources into sustainable energy (Tshemese et al. 2023). Despite the significant methane potential of lignocellulosic biomass, anaerobic digestion techniques have not efficiently utilized it to produce biogas. The efficient digestion of biomass during the anaerobic process is currently challenging with lignocellulosic substrate because of the complicated and refractory nature of the feedstock (Karrabi et al. 2023).
Several pretreatment procedures have been devised to change the structure and improve the digestibility of biomass for anaerobic digestion. Many pretreatment techniques, including biological, chemical, and physical ones, are employed (Abraham et al. 2020). Physical pretreatments, such as grinding and milling, are used to lower biomass particle size. Physical pretreatments will enhance biomass's surface area and improve biomass's digestion. The physical pretreatments do not produce harmful chemicals that hinder anaerobic digestion (Eswari et al. 2023).
Chemical pretreatments are divided mainly based on the kind of chemical into acid, alkaline, and oxidative. Sulfuric, hydrochloric, formic, and nitric acid are the most common acidic agents used in biomass pretreatment (Sarto et al. 2019). The primary mechanism of action of these treatments is the removal of the lignin or hemicellulose contained in the biomass. Biological pretreatment is ideal for biomass conversion utilizing fungus or bacteria because of its eco-friendliness and lower energy demand. Many fungi can rapidly break down cellulose and hemicelluloses by producing biological enzymes (Byun et al. 2020).
Several factors control anaerobic digestion, such as temperature, pH, and hydraulic retention time. Keeping these parameters at the appropriate level is crucial for the anaerobic digestion process to operate well over the long term. Temperature is a vital factor for the survival of microorganisms in anaerobic digestion (Singh et al. 2023). Microorganisms are susceptible to temperature gradients; lowering the temperature reduces the rate of volatile fatty acid synthesis, feedstock utilization rate, microbial metabolism, and biogas output (Kainthola et al. 2019).
The operational pH directly impacts the anaerobic digestion process and intermediate products, where a pH range of 6.8–7.4 is required to function correctly (Rajendran et al. 2020). The average duration spent by the substrate in the reactor is referred to as hydraulic retention time. While longer retention times need a bigger reactor volume and hence a less cost-effective design, shorter retention times expose the danger of active biomass washout (Kainthola et al. 2019).
Anaerobic digestion has several benefits over other biomass conversion technologies. It can process various organic materials, including agricultural waste, food waste, and sewage sludge, manure, seaweeds, and produces a renewable energy source that is cleaner and more efficient than fossil fuels (Farghali et al. 2021, 2023a; Lu et al. 2022; Peng et al. 2023). The process also helps to reduce greenhouse gas emissions and provides a valuable byproduct that can improve soil health (Karrabi et al. 2023). However, anaerobic digestion can be sensitive to changes in feedstock composition and operating conditions, which can affect the efficiency and stability of the process. The quality of the biogas produced can also vary depending on the feedstock and process conditions, affecting its suitability for different applications (Emebu et al. 2022).
Performance and share
The use of biomass energy is widely considered a promising option for transitioning from non-renewable to renewable energy sources. In this section, a comprehensive analysis of the energy market trends is conducted to better understand the current status of biomass energy and its potential for future expansion. According to Gielen et al. (2019), transitioning to more energy-efficient practices and utilizing renewable energy sources could potentially lead to a 94% reduction in anthropogenic carbon dioxide emissions by 2050. Renewable energy alone could contribute to a 41–54% reduction in carbon dioxide emissions. However, due to the larger scale and lower cost of non-renewable energy production, fossil fuels are projected to account for approximately 33.33% of the primary energy market. On the other hand, research by Zheng et al. (2021) on 30 provinces in China shows that a 0.01 increase in the renewable energy industry could reduce carbon dioxide emissions by 0.028–0.043%.
Today, the consumption of non-renewable energy still surpasses that of renewable energy. However, the Coronavirus disease 2019 (COVID-19) pandemic has significantly changed the global market for non-renewable and renewable energy sources (Farghali et al. 2023c). The data presented in Fig. 9 obtained from BP-Statistics (2022) highlight the statistics of global primary energy consumption between 2017 and 2021. The figure includes other renewable energy sources, such as wind, geothermal, solar, biomass, and waste, without considering cross-border electricity supply.
Global renewable energy consumption. The global renewable energy consumption continued to increase despite the impact of the Coronavirus disease 2019 (COVID-19) pandemic between 2019 and 2020, based on the data retrieved from BP-Statistics (2022). Hydropower is the most implemented among the renewables, possibly due to matured technology and considerably greater policy support. Conversely, non-renewables experienced a depletion, resulting from reduced human and economic activities during the pandemic. However, non-renewable energy remained the primary source, as depicted by considerably higher consumption than renewables between 2015 and 2020. Substitution of non-renewables by renewables is expected to take a long time, as indicated by the small increase in global renewable consumption each year
All non-renewable energy including coal, natural gas, oil, and nuclear energy, experienced a slight decline in global energy consumption between 2019 and 2020. The decrease in the non-renewables in 2020 was recorded as 6.25 EJ, 2.19 EJ, 17.96 EJ, and 1.02 EJ or 3.97%, 1.56%, 9.35%, 4.01%, 2.34%, and 9.64%, respectively. These reductions were the highest throughout the five years of the study as most non-renewables, except for coal, had increasing consumption before the pandemic. Non-renewables experienced an overall decrease of 27.42 EJ or 5.32% in world consumption between 2019 and 2020.
In contrast with non-renewables, the usage of hydroelectricity and other renewables increased by 0.94 EJ and 3.06 EJ or 2.34% and 9.64%, respectively, in 2020. The growth of the other renewable energies in 2020 was the smallest compared to 3.17 EJ, 3.21 EJ, and 5.11 EJ or 12.5%, 11.25%, and 14.68% for 2017–2018, 2018–2019, and 2020–2021, respectively. Regarding hydroelectricity, the most significant consumption growth was recorded in 2020 compared to previous years. In 2017–2018, the growth was 0.85 EJ or 2.18%, while in 2018–2019, it was 0.31 EJ or 0.78%. However, for 2020–2021, there was a decline of − 0.83 EJ or − 2.02%. The overall expansion of renewable primary energy consumption in 2020 relative to 2019 was recorded as 4 EJ or 5.56%. The gain of the overall renewables consumption in 2020 exceeded that of non-renewables by 31.42 EJ. The impacts of the COVID-19 pandemic were opposite in both sectors, showing that the demand for renewable energy was less affected by the global pandemic COVID-19 pandemic that halted global economic and industrial activities and caused a depletion in world energy consumption.
As stated by Jiang et al. (2021a), the impact of the pandemic on the fossil energy industry was more significant than on the renewable energy industry despite the restrictions in supply chains, showing the potential of renewable energy during the bottom of global energy demand. Although the demand for renewables has experienced obvious growth since the pandemic, they believed that renewable energy could not fully replace conventional energy any sooner due to the less adaptable production system. Lower flexibility in the renewable energy production system is disadvantageous as the production rate is not adjustable according to the current demand in the local and global market.
During the economic recovery phase in 2021, all types of energy sources experienced an increasing consumption except for hydroelectricity. The growth in the global consumption of other renewables was the most significant in 2021 compared to the study’s former years. On the other hand, the recovery of non-renewable primary energy consumption in 2021 was also the greatest when compared to the growth in the previous years, recorded as 9.03 EJ, 6.91 EJ, 10.04 EJ, and 0.88 EJ or 5.98%, 4.99%, 5.76%, and 3.60% for coal, natural gas, oil, and nuclear energy, respectively. Additionally, a new peak was achieved in the consumption of all non-renewable primary energies, except for oil, in 2021. This shows that lockdowns and restrictions of economic and industrial activities have no permanent positive effects on reducing non-renewable usage.
Although global renewable energy consumption continuously rises, the difference between the overall usage of non-renewables and renewables between 2017 and 2021 was considerably large as the current market size of renewable energy is smaller and less established than non-renewable energy. The data are represented in Table 7 to show the primary energy mix of 18 countries in descending order according to the overall energy consumption reported in 2021. The top 18 countries with leading primary energy consumption in 2021 are China, the USA, India, Russia, Japan, Canada, Germany, South Korea, Brazil, Iran, Saudi Arabia, France, Indonesia, the UK, Turkey, Mexico, Italy, and Australia.
Global primary renewable energy share in 2021 was recorded as 13.47%, while the shares of most countries are less than 20%. Prime factors influencing the global and national energy market trend are energy policies, national development plans, incentives or financial limitations, environmental requirements, supply–demand balance, technology constraints, and public awareness. Each country has a unique energy profile and outlook due to the differences in the abovementioned aspects. Xu et al. (2019) expected a high renewable energy industry growth rate in Asia and the USA, stimulated by economic expansion. Canada and Europe were expected to achieve continuous and steady development in the renewable energy industry because of their policies rather than economic advancement. Lastly, renewable energy usage in the Middle East, Latin America, and Africa will grow relatively slowly and is affected by the development plan and low demand.
China has been the biggest consumer of primary energy since 2009. As shown in Table 7, the country used 26.49% of the world's primary energy in 2021, where 85.05% of the energy consumed was from fossil fuels and nuclear energy. The market of non-renewable energy is well established in China, especially for coal which had the greatest share in 2021, recorded as 54.66% of the overall primary energy used by the country. Based on the data published by the International Energy Agency (2021), China significantly contributed to the total carbon dioxide emission from the growing demand for electricity and heat.
Despite that, renewable consumption is expected to grow continuously in China due to the goal of shifting to cleaner energy. Liu (2019) explained that energy insecurity caused by a rapid expansion of the economy and urbanization promotes the renewable energy industry. They stated that the national renewable energy is not fully utilized due to the lack of practicality and simplicity in energy laws, the well-establishment of conventional energy sources, and insufficient financial subsidies as well as irrational planning that causes abandonment of the existing solar and wind power. Based on the research by Zhang et al. (2020b), although urbanization may accelerate the growth of the renewables industry in China, the demand for fossil energy is raised simultaneously because of the nation's high level of fossil energy dependence. Their opinion about the outlook of renewable energy in China agrees with Fan et al. (2021) and Wang et al. (2021). The development of the renewable energy industry in China could be promoted by the increase in their gross domestic product to achieve their renewable portfolio standards and the country’s economic expansion.
The share of non-renewable energies in most countries in Table 7 falls in the 80–100% range in 2021. However, Canada and Brazil are the only exceptions with a relatively higher non-renewables share, recorded as 29.91% and 46.22%, respectively. The supply of renewables in Brazil made up almost half of the overall energy consumption in 2021. From the study by Pischke et al. (2019), Canada and Brazil had the highest overall intensity in the policies regarding renewable energy among the countries studied, including Mexico, the USA, and Argentina, between 1999 and 2015. Canada was reported to perform best in budgeting, while Brazil has the most effective enforcement of relevant laws and monitoring.
Hydropower holds a considerable share of the renewable energy market in many countries. One example is China, with a comparable share of hydroelectricity and other renewables, recorded 7.77% and 7.18%, respectively, of the national energy usage in 2021. According to Li et al. (2018c), in 2015, China's hydropower capacity and growth rate were the greatest globally. However, the abandonment of the existing hydropower plants in Sichuan escalated from 2013 to 2020 because the hydropower generated was not fully utilized in the served regions. Served regions have lower demand due to less developed and diverse economies. Moreover, thermal power is deemed more capable of ensuring power security which inevitably promotes the use of coal-fired power generation in China. The abandoned capacity was estimated at 35 billion kWh or 8.64% of the overall electricity produced in 2020.
Fan et al. (2021) stated that more than 50% of China's overall renewable energy production is hydropower. The rapid expansion of hydropower is to help the country to accomplish the renewable portfolio standards targets in 2020. Nonetheless, some provinces in China relied on cross-border hydro and non-hydroenergy transfer due to ununiform development. In the case of China, renewable energy expansion is slowed down by socio-economic factors, despite the number of hydropower plants, small or big, in the country, increased dramatically over the years.
Hydropower accounted for 6.45% of the primary energy consumption in Russia in 2021, which is higher than the share of the other renewables by 6.26%. Voropai et al. (2019) reported that the expansion of hydropower in Russia was expected to be pushed by the cross-border electricity supply, which brings about economic benefits, exploitation of resources, and rural or suburban development, as well as the advancement of transport infrastructures. Nevertheless, as discussed by Bogoviz et al. (2020), the growth of electricity production from nuclear energy was expected to grow at a rate of 1.2–1.3 times hydroelectricity due to the “Strategy of the Energy Development of Russia” by 2035, which possibly hinders the development of hydropower in Russia. Apart from that, environmental conditions, defective and old equipment, and the lack of assessment of the potential of hydropower as an important facility to utilize natural resources and untouched areas could slow down hydropower development in the nation.
The energy situation in India is crucial to the world's energy statistics and anthropogenic carbon dioxide emission since the country has the second largest population. With such a significant impact, there is a high potential to deploy renewable energy into the country. Referring to Table 7, the hydroelectricity share and the other renewable energies share of India in 2021 were comparable, with a difference of 0.79%; according to Kumar and Majid (2020), the share of wind power, solar energy, bagasse, small hydropower, biomass, and wastes in Indians’ renewable electricity generation from 2017 and 2018 was 51.71%, 25.40%, 11.63%, 7.55%, 3.34%, and 0.35%, respectively. They also reported that several problems appeared to delay the deployment of renewable energy in India, including national policies, incorporative institutions, financial plans and subsidies that favor the conventional energy industry, and technology limitations which mainly account for environmental impacts and profitability. Other hindrances are the insufficiently trained workforce as well as the lack of public awareness and support for the use of renewables.
Hydropower has been the leading renewable source of electricity generation to lower global anthropogenic carbon dioxide emissions, but the environmental impacts of constructing hydropower infrastructures are unneglectable. Li et al. (2018c) stated that hydropower projects could destroy the local ecosystems and natural habitats due to deforestation as well as affect the ecological and geological environment by posing changes to the ecological structure. According to Bogoviz et al. (2020), hydropower projects adversely affect the underwater environment and organisms despite the relatively economical energy source. In addition, a study conducted by Pata and Kumar (2021) examined the effectiveness of hydropower in reducing carbon dioxide emissions from coal-fired plants in China and India. The study found that while the environmental impacts of coal consumption in both countries were significant, hydropower may not be the most effective solution due to the potential disturbance of river and lake water flow, as well as the destruction of land and natural reserves during the construction of hydropower stations.
Besides, the planning and construction of hydropower plants have high complexity. Li et al. (2018c) discussed considerations in hydropower projects. Flooding of a large area of land affects the residents. Relocation of homes leads to additional costs and the need to offer economic compensation and ensure social development. Bogoviz et al. (2020) stated that building hydropower plants and projects takes at least 4–5 years. The long duration of a hydropower project could affect the investors’ interests. Plus, the efficiency of carrying out hydropower projects depends on environmental conditions.
Biomass energy is a potential candidate in the era of the global energy transition. The carbon footprint of biomass burning is high, but the effect is deducted from using carbon dioxide in regrowing raw materials. However, solar energy appears to be a strong competitor of biomass energy in the renewables market. Even so, in the construction and operation process of solar power plants, carbon dioxide emission, and non-renewables consumption are remarkable.
Wu et al. (2021) reported solar power plants' considerably high carbon footprint based on calculating non-renewable energy investment in energy delivered by a pilot solar plant. Their research disclosed that the electricity yield from the pilot solar plant is 1.6 times lower than the non-renewables consumed. They identified several areas of a solar power plant that consume non-renewable energy and emit carbon dioxide, as illustrated in Fig. 10.
Non-renewable energy consumption and carbon dioxide emission in a pilot solar plant. The data show a similar composition in the pie chart, implying a close correlation between the two elements. Solar collectors’ field accounts for the highest percentage, recorded as 31.50% and 30.63% for non-renewables consumption and carbon dioxide emission. The dependence of the pilot solar plant on non-renewables is considered significant, followed by the contribution to the overall footprint of the plant. Additionally, electricity yield is less than the non-renewable energy consumption by a factor of 1.6. This implies the lack of sustainability of the pilot solar power plant for larger scale application and long-term operation despite the renewability of the energy source and production. The data retrieved from Wu et al. (2021)
In Fig. 10, seven sections in the pilot solar plant studied, including solar collectors’ field, energy storage system, heat exchange system, test base, plant maintenance and operation, services, and turbo-generator subsystems, were found to be non-renewable energy intensive. The share of solar collectors’ field was the greatest, recorded as 31.50%, followed by turbo-generator subsystems, maintenance and operation of the plant, heat exchange system, and services with a share of 18.50%, 15.67%, 11.08%, and 11.02%, respectively. On the other hand, the share of an energy storage system and test base was below 10%, recorded as 7.39% and 4.84%. Turbo-generator subsystems were further categorized into an electric subsystem, thermal control subsystem, chemical water treatment subsystem, heat engine subsystem, and others. The shares of non-renewables consumption were 4.30%, 2.57%, 1.65%, 8.06%, and 1.92%, respectively.
With considerable non-renewable energy consumption, carbon dioxide emission from solar power plants is also significant. Similarly, solar collectors’ field holds the major share of the carbon footprint of the solar power plant, recorded as 30.63%, followed by turbo-generator subsystems, electric subsystems, thermal control subsystems, chemical water treatment subsystems, heat engine subsystems, and others with a share of 18.88%, 4.36%, 2.56%, 1.67%, 8.14%, and 2.15%, respectively. Maintenance and operation of the plant, services, heat exchange system, test base, and energy storage system were 13.45%, 12.05%, 11.89%, 8.17%, and 4.94%, respectively.
Figure 11 compares non-renewable energy usage and carbon dioxide emissions from a pilot solar power plant and three coal-fired power plants with different electricity production capacities. The three coal-fired power plants included in the comparison are located in the USA, with a capacity of 360 MW, the UK, with a capacity of 500 MW; and China, with a capacity of 2 × 660 MW. The electricity production capacity of the studied solar power plant was recorded as 1.5 MW, corresponding to 0.42%, 0.30%, and 0.11% of the coal-fired power plant capacities of 360 MW, 500 MW, and 2 × 600 MW, respectively. Despite the big difference in the production capacity, the non-renewable energy usage of the solar power plant was 5.76 MJ/kWh, equivalent to 46.41%, 58.06%, and 55.17% of the coal-fired power plants. Furthermore, carbon dioxide emission of the solar power plant was reported as 633 g carbon dioxide/kWh. Compared with the coal-based power plants in terms of carbon dioxide emission per electricity produced, the generation of anthropogenic carbon dioxide of the solar power plant is 72.01%, 61.94%, and 63.94% of the three coal-fired power plants.
Non-renewable energy usage and carbon dioxide emissions from a pilot solar power plant and three coal-fired power plants. Based on the data retrieved from Wu et al. (2021), the electricity production capacity of the pilot solar plant is 1.5 MW which is 0.42%, 0.30%, and 0.11% of the coal-fired power plant capacities of 360 MW, 500 MW, and 2 × 600 MW, respectively. Non-renewable energy usage of the pilot solar plant was 5.76 MJ/kWh, which is 46.41%, 58.06%, and 55.17% of the coal-fired power plants. Due to high non-renewables consumption, the carbon emission of the pilot solar plant also exceeds that of coal-fired power plants. A comparison between the two sets of data shows that the pilot solar plant's energy intensity and carbon footprint are higher than coal-fired power plants. Efforts are needed to lower the dependence on non-renewables in solar power plants to enhance sustainability for long-term implementation
Wu et al. (2021) used the coal-fired power plant with a capacity of 2 × 600 MW in China as a reference to determine the sustainability and carbon dioxide emission reduction in the pilot solar power plant. The calculation shows that, with the implementation of solar power, 44.83% of non-renewable energy consumption was eliminated, while carbon dioxide emission was lowered by 36.06%. Compared to the 360 MW and 500 MW coal-fired plants, the decrease in non-renewability usage is 53.59% and 41.94%, while carbon dioxide emission reduction is 27.99% and 38.06%, respectively. Solar power applications are more difficult for large-scale electricity production than non-renewables, especially fossil fuels. The installed quantities, land areas, and related costs shall be increased to fulfill the increasing demand. Importantly, non-renewable energy investment in energy delivered and the polluting effect, particularly the carbon footprint of a solar power plant, are considered where the values account for more than 50% of a coal-based power plant.
Another competitor of biomass in the renewable energy market is wind energy. The energy source has a lower carbon footprint, but limitations still hinder its widespread application worldwide. According to Nazir et al. (2019), significant impacts on wildlife and the environment have not been identified. However, noise pollution was reported as a major issue that could induce human health problems such as anxiety, sleeping and hearing disturbance, and probable damage to the vestibular structure. Also, the possibility of changing the heat and climate of a region as well as the local humidity, was mentioned, but the actual effects are uncertain. From the low carbon footprint, the sustainability of the energy source, and insignificant side effects on the environment, wind energy is seemingly an excellent substitute for non-renewables.
Still, wind energy development faces a similar challenge to solar energy: the uncertainty in the sources. Nazir et al. (2019) stated that wind energy production is not constant because of the uncertainty in wind supply. In accordance with Veers et al. (2019), the controllability and predictability of power converted from wind or solar energy are two pivotal factors to ensure the functionality, dependability, and stability of the transmission infrastructures or grid. The control systems must be able to confront the variation in the sources and the demand.
Proper survey and planning are essential to identify the potential location of a wind power plant because the location is greatly dependent on the geological properties and the wind advantages, which could be seasonal. Similarly, solar power plants shall consider the strength of sunlight throughout a day or a season. Thus, potential areas for power plants of wind energy and solar energy are limited by environmental conditions. Consequently, the formerly mentioned problem regarding the supply–demand balance in China’s hydropower industry could appear where the plants are built to serve nearby low-demand areas.
Moreover, the expansion and advancement of wind, hydropower, and solar energy are affected by the imperfections of transmission infrastructures to support current and future power supply. This adds to the importance of developing an electricity grid that can transmit large voltage to the end users located far away. As Wei and Chen (2019) explained, the abandonment of hydropower, wind energy, and solar energy is occasioned by the incompatibility of grid development with the supply capacity of the renewables to meet the actual consumption requirement. They reported that the percentage of abandoned wind power production in China was the highest between 2011 and 2017, recorded as 17% in 2012 and 2016, while the average percentage was 13.57%.
Based on the data disclosed by Jiang et al. (2021b), the abandonment of China hydropower increased from 515 hundred million kWh in 2017 to 691 hundred million kWh in 2018 by 176 hundred million kWh or 34.17%. In contrast, abandoned wind power was reduced by 142 hundred million kWh or 33.89%, from 419 hundred million kWh to 277 hundred million kWh between 2017 and 2018. Solar power experienced slight depletion in abandonment recorded as 18.1 hundred million kWh or 24.79% from 73 hundred million kWh to 54.9 hundred million kWh. The phenomena happen due to inadequate demand when compared to the supply quantity.
In comparison with other renewable energies, biomass energy has a few advantages. Foremostly, the source of biomass energy is diverse and renewable. Malico et al. (2019) stated that solid biomass such as wood chips, barks, and nut shells is commonly found as feedstock for biomass energy generation. Furthermore, biochar and biomass treated with torrefaction have gained popularity in some industries that strike for higher energy efficiency, including the iron and steel industries. According to them, pre-treatment helps reduce the cost and difficulty in transporting and keeping the fuel.
Waste to energy is an attractive option that could only be offered by biomass energy. In this process, wastes from various sources are used as feedstocks for energy production. Kaza et al. (2018) stated the positive effects of urbanization and the level of income or gross domestic product of a country on the waste production rate. By 2050, global waste production was expected to reach 3401 Mt/year, with East Asia and the Pacific holding the most significant share of 20.99%. Their data also revealed that 36.7% of global waste goes into landfills, 33% is openly dumped, 11% undergoes incineration, and 13.5% is recycled. Improper waste management could lose the opportunity to recover energy from non-recyclable wastes.
Municipal wastes are polluting and not recommended for open dumping. Perrot and Subiantoro (2018) reported a small difference of 5.3 PJ between the coal consumption, recorded as 25.5 PJ, and the annual recoverable energy from municipal wastes in 2016, recorded as at least 30.8 PJ, in New Zealand, indicating the potential of municipal wastes in satisfying the coal demand in the country. Plastic which makes up 12.1% of municipal wastes in New Zealand, contains the highest recoverable energy, 35 MJ/kg. Textiles, organic wastes, timber, and rubber have an energy content of 19 MJ/kg, 3 MJ/kg, 6 MJ/kg, and 14 MJ/kg, respectively. Paper, nappies, and sanitary have an overall composition of 15.3% and an energy content of 16 MJ/kg. Agriculture wastes, mainly the unwanted parts of crops, including straw, husk, shoot, leaves, bagasse, peel, and shell, can be used as raw materials for energy generation. Sivabalan et al. (2020) explained that the conventional way to tackle agricultural waste is to either burn it or leave it for natural soil erosion, leading to no exploitation of recoverable energy.
Biomass energy is sourced from solid biofuel, liquid biofuel, and biogas. According to Sindhu et al. (2019), solid biofuel or biochar is manufactured from biomass's thermal decomposition to improve the material's carbon content. The application of biochar can be found in soil conditioning and fertilizing, which is essential for increasing soil carbon content and lowering carbon dioxide concentration in the atmosphere. Liquid biofuels such as biomethanol, bioethanol, and biobutanol generally have high octane numbers and low carbon emissions, which make them a promising substitute for fossil fuels in transportation. They further explained that biogas comprising 50–85% methane is generated by breaking down organic matter anaerobically, and the wastes from the process are added to the soil for quality enhancement. Another promising source for biomass energy production is food waste because of starch, cellulose, hemicellulose, organic acids, lipids, lignin, and protein, as stated by Kour et al. (2019). Energy recovery efficiency from various wastes is pivotal in secondary pollution prevention, cost reduction, and process scale determination.
Besides the variety of sources, biomass is advantageous by possessing zero carbon footprint. Research by Beagle and Belmont (2019) claimed that 20% biomass integration in the power plant, or co-firing power plant, could achieve much lower carbon dioxide emissions than a coal-fired power plant. The reduction in carbon dioxide emission was between 1 and 15%. Additionally, the depletion of carbon dioxide emission lies in the range between 54 and 73% in biomass-based power plants. Ilari et al. (2022) revealed that using agricultural wastes as feedstocks could avoid 49.76% of carbon dioxide emissions from a process using wood chips from silviculture.
Since biomass source comprises mainly vegetation, the bioenergy production system is considered carbon neutral and has higher flexibility. The production system of any energy must be adjustable according to the availability of the sources and the consumers’ demand. The case of solar energy- and wind energy-based production indicates the importance of the controllability of energy feedstock. Energy input or feedstock, namely sunlight, and wind, is uncontrollable and variable, leading to inconsistent output and higher requirements of infrastructures and control systems. Actual demand shall also be considered in energy production to maintain the balance between supply and demand. The excess energy supply over demand is prone to appear in the hydropower, solar, and wind energy industries because the production capacity is not adjustable. Although the sources are accessible, oversupply could cost the energy companies and lead to the decision to abandon their power plants.
Next, integrating biomass into the existing power plant is more accessible than other renewables (Farghali et al. 2023b). The effectiveness of integrating biomass into fossil fuel-based power supply systems was previously elaborated. Fan et al. (2018) studied biomass integration in manufacturing synthetic natural gas by coal gasification. They stated that integration posed significant improvement in energy conversion efficiency, which helps in primary energy saving, recorded as at least 15% with 45% of biomass blending, saving steam usage and increasing plant productivity. Overall, integrating biomass in existing fossil fuel power plants could effectively improve the environmental friendliness of the power generation process. Biomass blending in fossil fuels for power production is facilitated by the similarity between the fuel properties, which leads to higher compatibility with the existing system than other renewables.
Biomass energy is increasingly popular due to the global energy transition from non-renewables to renewables. However, the status of biomass energy varies in different sectors. To study the current demand for biomass in the renewables market, the following discussion regards the role of renewable energy and biomass in three prime energy-consuming sectors: electricity, transport, and heat.
Research by Rahman (2020) investigating China, the USA, India, Japan, and the UK concluded that electricity consumption poses significant environmental impacts via high carbon dioxide emission due to high electricity demand in economic activities. The electricity demand is anticipated to rise rapidly due to the shift toward electrification, which is expected to effectively reduce overall fossil fuel consumption in electricity generation. As a result, a global transition to renewable energy sources is necessary for electricity production. At the same time, improving the efficiency of the existing production and transmission systems is essential to achieve a lower carbon footprint.
In the transport sector, biofuel is promoted as the future substitute for conventional fossil fuels. Ebadian et al. (2020) explained four main factors promoting global production and biofuel use: energy security, rural expansion, environment conservation, and job opportunities. In the USA, Brazil, China, and India, biofuel demand is attributed to sufficient and low-cost raw materials and to develop energy security and rural districts. The biofuel industry in European Union, Japan, and South Korea is motivated by conserving the environment and lowering or eliminating carbon emissions of transport use using clean energy. On the other hand, South Africa relies on the biofuel industry for economic growth and to provide more job opportunities to help develop rural areas and agricultural sectors.
Heat production is realized by either burning fuels or electrification. Primary energy is converted to electrical energy, which is later sourced by electrical heating equipment used in residential and industrial activities. Despite lower carbon dioxide emission, Las-Heras-Casas et al. (2018) explained that biomass is deemed less cost-effective in temperate winter zones and heating with biomass could have higher emission of carbon monoxide, non-methane volatile organic compounds, and particular matter PM10 than conventional fossil fuels. Bertelsen and Vad Mathiesen (2020) reported that the transition from coal and oil to natural gas and biomass for domestic uses led to a decline in carbon dioxide emission by 189 MT or 28% from 683 to 494 MT between 1990 and 2015. They also mentioned that the energy transition is affected by the dependence of residential heating on the infrastructure, and the technologies used in the existing systems for different fuels are not interchangeable.
Industrial heating processes are identified as promising areas to promote carbon dioxide emission reduction due to their enormous contribution to global emissions. Malico et al. (2019) stated that since biomass is a by-product of some industrial processes, bioenergy generation is more feasible than the other processes where fossil fuels are more accessible than biomass. Therefore, investment cost plays a pivotal role in encouraging the use of biomass as feedstocks for industries with low biomass availability. Based on the study by Rehfeldt et al. (2020), the potential reduction in carbon dioxide emission in steam production or furnaces is the highest, recorded as 50%, followed by glass, clinker, and lime production at 40%, 39%, and 35%, respectively, besides refineries, construction sectors, and iron industry. Moreover, the biggest challenges to promoting cleaner energy, such as biomass and electricity, are the low competitiveness of clean technologies from economic aspects and the incompatibility of the existing infrastructures that use biomass and electricity as feedstocks. Similarly, Pisciotta et al. (2022) stated that biofuel properties and infrastructure compatibility shall be considered.
By referring to World Bioenergy Association (2021), solid biomass, including wood chips, pellets, and other conventional feedstocks, has the highest share, recorded as 85% in the overall biomass supply of 2019. The share of liquid biofuel, municipal waste, biogas, and waste share was 8%, 3%, and 2%, respectively. The application of gaseous and liquid biofuel is still less than that of biomass despite being the most anticipated replacement of fossil fuels to achieve a sustainable and carbon–neutral future. This could be caused by the high cost of large-scale production and the limitations of the existing technology and infrastructures to support a higher percentage of biofuel implementation. Besides, the deployment of municipal waste and industrial waste into the biomass energy industry must consider preventing secondary pollution through proper treatment and technological advancement.
According to the report by Renewable Energy Policy Network for the 21st Century (2022), non-bioenergy holds a major share in electricity generation, transport, and industrial heat production in 2020, which are 97.6%, 96.5%, and 90%, respectively. Traditional bioenergy was only found in providing heat to buildings, and the share was recorded as 25.6%, which is higher than modern bioenergy by 20.4%. Conversely, traditional bioenergy has a 0% share in electricity production, transport use, and industrial heat generation, while modern bioenergy in the three sectors was 2.4%, 3.5%, and 10%, respectively. Traditional bioenergy, which is generated from the direct burning of biomass, is less efficient than modern bioenergy, and therefore, traditional bioenergy is not preferred in industrial processes. The biomass market in the three sectors is smaller when compared to the overall demand for non-bioenergy, including non-renewables and other types of renewables (International Renewable Energy Agency 2022).
To study biomass demand trends in electricity production, heat generation, and transport use, statistics of the global energy industry and renewables market in the three sectors are obtained from World Bioenergy Association (2021) and discussed in the remaining section. Non-renewables generally have a bigger market than renewables in the three sectors. Heat generation for industrial activities and buildings is mainly sourced from fossil fuels, particularly natural gas and coal. As reported, the share of oil, natural gas, and coal in global heat production in 2019 was 3%, 41%, and 45%, respectively, which is almost 90% of the overall heat produced. On the other hand, renewables and nuclear energy account for 11% and 0.17% of global heat production. In the production of electricity, renewable energy demand is lower than fossil fuels since only 27% of global electricity in 2019 was produced with the use of renewables, while oil, natural gas, and coal held a share of 3%, 23%, and 37%, respectively, which is equivalent to 63% in total. In addition, the use of oil as a transport fuel is still prevalent. The share of oil in global transport fuel in 2019 was 92% compared to 3% of biofuel.
Figure 12 depicts the global renewable electricity, transport, and renewable heat between 2015 and 2019. Electricity production from renewable energy rose from 5771.71 TWh in 2015 to 7310.10 TWh in 2019 by 1538.39 TWh or 26.65%. Among different renewables, the solar energy industry experienced the largest gain between 2015 and 2019, 440 TWh or 173.23%, from 254 to 694 TWh. This is followed by wind energy, which increased consumption from 834 to 1427 TWh by 593 TWh or 71.10%. Next, the use of biomass in generating electricity grew from 620 TWh in 2015 to 768 TWh in 2019 by 148 TWh or 23.87%, while that of geothermal energy grew from 80.7 TWh to 91.1 TWh by 10.4 TWh or 12.89%. Consumption of tide energy was reduced slightly by 0.01 TWh or 0.99%.
Global renewable electricity, transport, and renewable heat between 2015 and 2019. The data show that biomass energy for electricity production is severely lacking compared to hydropower and solar power in terms of the overall generation and production expansion between 2015 and 2019. Biomass energy dominates global transport fuel and heat production with continuous demand increase in the studied period. Renewable electricity experienced a significant increase in transport fuel, implying the rising competition with biofuel. Biomass energy had the most significant gain in 2019, recorded as 95.94% compared to other renewable fuels in the heat production industry. Biomass energy demand in transport and heat production industries is expected to continue rising due to the higher compatibility with the existing system than other renewables—data sourced from World Bioenergy Association (2021)
The expansion of hydropower consumption for electricity production between 2015 and 2019 was smaller than biomass, recorded as 347 TWh or 8.71%. Consumption of hydropower in 2019 relative to 2015 was lower compared to biomass, solar, wind, and geothermal energy. Still, hydropower had the highest consumption throughout the five years. This shows the well-establishment of the hydroenergy industry in producing renewable electricity. Conversely, the proportion of biomass in total renewable electricity production decreased by 0.23%, from 10.74% to 10.51%, between 2015 and 2019.
The share of hydropower in the renewable electricity market was 59% in 2019. Wind energy was ranked second with a share of 20%, followed by biomass energy with 11% of overall renewable electricity produced in 2019. The share of solar energy was recorded as 9%. The difference between biomass and solar energy was 2%, while that between biomass and wind energy was 9%. Between 2015 and 2019, the growth of the biomass market in the renewable electricity sector is lower than wind energy and solar power. Like any other renewables, the competitiveness of biomass in this sector depends on the policies to support the use of biomass, integration of biomass energy in conventional fuel-based power plants, and investment in developing biomass-based power plants.
Total renewable energy in global transport expanded from 3.57 to 4.4 EJ by 0.83 EJ or 23.25%. Consumption of transport biofuel rose from 3.28 to 3.99 EJ by 0.71 EJ or 21.65%, while renewable electricity escalated from 0.29 to 0.41 EJ by 0.12 EJ or 41.38% between 2015 and 2019. Renewable electricity growth is higher than biomass by 19.73%. This significant gain in renewable electricity shows the potential of the energy to compete with biomass as a renewable transport fuel in future.
Biomass share decreased slightly from 91.88 to 90.68% by 1.19%, attributed to the rapid growth of the renewable electricity market for transport. Although electrolysis has gained increasing interest recently, biomass still held the most share in the renewable transport fuel sector in 2019. Mixing biofuel with conventional fuel has been carried out to improve the sustainability of the transport fuel. This may explain the dominance of biomass in the renewable transport sector. According to Sindhu et al. (2019), there is no alteration of the engine needed to use a mixture of gasoline and bioethanol with a concentration at most 85% by volume. 2, 5–dimethylfuran is also a promising biofuel which was disclosed in the past study that 5% of the biofuel mixing with gasoline has similar quality with gasoline. However, low implementation compared to fossil fuels may be caused by the high cost of production.
Total renewable heat produced in 2019 was 1.662 EJ which is higher than that in 2015, recorded as 1.391 EJ by 0.271 EJ or 19.58%. Renewable heat generated by biomass increased by 0.26 EJ or 19.26% from 1.35 EJ in 2015 to 1.61 EJ in 2019. On the other hand, heat production by solar and geothermal energy remained relatively constant compared to biomass energy. Nevertheless, solar based-heat production in 2019 was twice that in 2015, recorded as 0.001 EJ and 0.002 EJ, respectively. The increase in heat production by geothermal energy was 0.01 EJ which is 25% of that in 2015. Moreover, the growth in biomass application for producing heat accounted for 95.94% of the overall expansion in 2019.
Table 8 compares the consumption of renewables in electricity production, transport, and heat production in 2019 by continent. European Union–28 has the largest biomass share in renewable electricity, which is 23.9%, compared to Africa, the Americas, Asia, Europe, and Oceania, with a biomass share of 1.12%, 8.57%, respectively, 8.67%, 17.5%, and 4.64%, respectively. The share of hydropower of all continents was the highest, which is 80.33% in Africa, 64.73% in the Americas, 60.92% in Asia, 47.70% in Europe, and 46.82% in Oceania except for European Union–28. The renewable electricity industry was dominated by wind power in the European Union–28 with a share of 35.36%, higher than hydropower by 6.38%. Solar energy and biomass had an immediate difference in the share, where the share of both energies was averagely lower than 10% in 2019.
Americas had the biggest biomass share in renewable transport in 2019, recorded as 98.89%, followed by the European Union–28 with 89.20% of biomass share. The biomass share of Asia, Europe, and Oceania was 75.36%, 79.38%, and 50.00%, respectively. In Africa, the share of biofuel was recorded as 0%, indicating the strong dependence of the continent on conventional fossil fuels for transport use. Overall, the distribution of biomass market is not evenly distributed even though biomass is well-established in the sector compared to other renewable fuels.
Biomass remained the biggest contributor to renewable heat generated throughout the 5 years. Biomass share in the heat production sector was 97%, while the share of geothermal energy was 3%. The use of solar energy in global heat production is negligible compared to biomass and geothermal. Thus, biomass is the most popular renewable energy source in the heat production sector. However, gas and coal have a greater demand and consumption than biomass in the world of heat production, as aforementioned.
Americas, Asia, Europe, and European Union–28 used majorly biomass as a renewable heat source. The share of biomass for renewable heat generation in the Americas, Asia, Europe, and the European Union–28 was between 96 and 100% in 2019. In contrast, Africa and Oceania had 0 EJ of renewable heat produced in 2019. This implies that the two continents have very low renewable heat demand and depend greatly on non-renewables in heat generation. Similar to the case of the transport field, the demand for biomass, or generally renewables in heat production, is uneven in the world.
As stated by International Renewable Energy Agency (2022), to maintain the increase in the world temperature below 1.5 °C, the supply of primary biomass should increase from 55 to 99 EJ by 44 EJ or 80%, followed by a growth of 109 EJ or 71.24% to 153 EJ before 2050. Simultaneously, a reduction in traditional biomass energy usage and an increase in modern biomass energy supply was expected. In the goal of a 1.5 °C pathway, overall biomass demand would rise significantly by a triple and quadruple by 2030 and 2050, respectively. Modern solid biomass was expected to have the biggest share, but the increase in demand would slow down after 2030. The demand for liquid biofuel and biogas and their use to generate heat and electricity is expected to grow continuously by 2050. Liquid biofuel would have the second biggest share in biomass products by 2050, while the share of the other biomass products, including biogas and biomethane, bioheat, bioelectricity, and chemical feedstocks, was expected to be comparable.
In summary, biomass has a high potential to be a substitute for non-renewables in future energy production process due to its zero carbon footprint, easier integration with existing energy production systems, and renewability. However, challenges such as insufficient effective policies, high cost or financial barriers, significant carbon footprint in the supply biomass, instability of the supply chain, and competition with other renewables are to be tackled to ensure the widespread application of biomass in the three prime energy sectors discussed previously.
Application of biochar in renewable energy
Solid biomass, including raw biomass and biochar, has widespread application compared to liquid and gaseous biofuel. According to Kazemi Shariat Panahi et al. (2020), several prime physical characteristics of biochar, such as porosity, particle size distribution, density, and mechanical strength, are to be considered in biochar production. Different property is targeted based on the design purpose of biochar. For example, higher porosity and density are preferred for solvent or gas adsorption and decolorization. On the other hand, as mentioned by Yaashikaa et al. (2020), the chemical properties of biochar are dependent on the concentration of carboxyl, hydroxyl, amine, amide, and lactone on the material surface, which could be affected by other factors, including porosity, pH, and surface area. Types of thermal treatment and process conditions are pivotal to altering the above-mentioned physiochemical properties to produce biochar with desired properties.
Common thermal treatment methods used to convert raw biomass to biochar include pyrolysis, gasification, and torrefaction, which utilize heat energy to improve the quality of the fuels. In many studies on biochar, carbon content is the main indicator of biochar quality. As stated by Wang et al. (2020b), oxygen availability and other prime reaction parameters could alter the quality and quantity of biochar. Therefore, biochar's chemical properties depend on the selected thermal treatment process, as each method has different reaction conditions. Anand et al. (2022) explained that a larger carbon-to-hydrogen or oxygen ratio is preferred due to higher structural stability. Thus, carbon content is a pivotal parameter for coal co-firing purposes.
Pyrolysis occurs in an oxygen-limited environment and can be divided into slow and fast pyrolysis. Referring to Wang et al. (2020b) and Yaashikaa et al. (2020), fast and slow pyrolysis has a few differences. Foremostly, the major product in fast pyrolysis of biomass is liquid bio-oil with biochar and syngas synthesized as the minor products. In contrast, biochar is the desired product in the slow pyrolysis of biomass. This difference is attributed to the variation in heating rate, which is at least 100 °C/min or up to 1000 °C/min for fast pyrolysis to facilitate a relatively more rapid decomposition of biomass than slow pyrolysis with a heating rate of 5–7 °C/min. The low heating rate of slow pyrolysis can be achieved by using a longer residence time of a least one hour for raising the temperature to pyrolysis temperature, which often lies in the range of 300–700 °C. Higher pyrolysis temperature, longer process duration, and slower heating are pivotal factors affecting biochar quality in slow pyrolysis.
As previously discussed, biomass is sourced from various materials, including vegetation, municipal wastes, and food wastes. Yaashikaa et al. (2020) stated that vegetation is preferred due to the higher calorific value, dryness, density, and lower debris, dampness, and porosity. These are the opposite of municipal wastes. Therefore, the physiochemical properties of biochar, production cost, and pyrolysis duration using municipal wastes and vegetation are dissimilar. Lu et al. (2020) suggested the potential of co-pyrolysis of different municipal wastes to produce biochar in advancing low carbon footprint and utilization of municipal wastes. However, the idea lacks practicality due to variable feedstock composition, possible process contamination, high cost, and municipal waste pyrolysis environmental impacts.
Research by Yuan et al. (2020) examined the differences between slow pyrolysis and fast pyrolysis in producing biochar from walnut shells based on three important aspects, including forming free radicals, oxygen-containing functional groups on the biochar surface, and graphite crystalline structure. According to them, the breaking of intramolecular covalent bonds produces free radicals. According to Yaashikaa et al. (2020) and Kumar et al. (2020a), slow pyrolysis of vegetation, which includes cellulose, hemicellulose, and lignin, results in the formation of biochar through various mechanisms, such as depolymerization, isomerization, dehydration, aromatization, decarboxylation, and charring. Breaking the β–O–4 linkage in lignin is the primary source of free radicals in biomass pyrolysis. Depolymerization of cellulose and hemicellulose occurs through the cleavage of strong covalent bonds in the molecules, producing levoglucosan and oligosaccharides. Syngas and bio-oil are synthesized through other reactions, such as the dehydration of levoglucosan and the further decomposition of hemicellulose.
Partial combustion is carried out with reduced air to facilitate gasification. Gasification usually occurs at a higher temperature range than pyrolysis, reported as 700–1000 °C by Wang et al. (2020b) and 650–900 °C by Yaashikaa et al. (2020). Biochar is produced when carbon-containing biomass is decomposed into gases comprising carbon monoxide, carbon dioxide, methane, hydrogen gas, and light hydrocarbons. Like fast pyrolysis, biochar is the by-product of the gasification of biomass. Therefore, the focus is often placed on the improvement in purity and quantity of syngas produced instead of biochar. This is in accordance with Yao et al. (2018), who stated that the development of models to gain better simulation of biomass gasification primarily considers syngas' quality instead of biochar production.
Torrefaction is divided into oxidative, steam, and wet or hydrothermal carbonization. This relatively new biomass thermal treatment technique generates biochar as the only product under vacuum conditions. In wet torrefaction, municipal wastes and other biomass with considerable moisture content are used as feedstocks to produce hydrochar. Biochar is in a relatively dry form as compared to hydrochar, which consists of a liquid phase. Pyrolysis is the most efficient and fastest way to produce biochar among all treatment methods discussed.
Biochar for electricity generation in microbial fuel cell
One proposed solution for biochar in producing renewable electricity is combusting the feedstock to generate steam. Direct biochar combustion is often applied in coal co-firing plants where biochar is blended with coal as feedstock. Based on the research by Anand et al. (2022), biochar has small differences from coal in terms of physical and chemical composition. Hence, substituting coal with biochar as feedstock in coal-firing plants for electricity production has a high potential for global carbon dioxide emission reduction. Nonetheless, several challenges to increasing the coverage of biochar usage in electricity generation via direct combustion were also reported, particularly in India. The challenges include inadequate biomass supply due to outdated mechanisms of agriculture waste collection, high cost of transporting and handling, insufficient infrastructures, unawareness of the market, and low investment interest.
On the other hand, using biochar as electrodes of a microbial fuel cell in electricity production is based on redox reactions. The occurrence of a redox reaction involving the oxidation of organic compounds releases electrons and forms a current. Unlike the direct combustion of biochar for electricity production, no heat energy is consumed using microbial fuel cells. Figure 13 illustrates a simple schematic diagram to represent the working principle of a microbial fuel cell.
Microbial fuel cell principals. In a microbial fuel cell, oxidation and reduction occur simultaneously to produce electricity. Organic substrates are degraded and oxidized to form carbon dioxide and protons during microbial activities in an anode chamber. In the cathode chamber, no microorganisms are present, and protons diffuse through the barrier to be reduced to water. The cathode chamber is usually open to the atmosphere for continuous oxygen supply. Anode properties regarding porosity, biocompatibility, toxicity, and others are crucial to maintaining microbial activities and consistent electricity production. CO2 refers to carbon dioxide
A microbial fuel cell consists of an anode and a cathode. Oxidation of organic substrates occurs at the anode, where the organic compounds undergo decomposition to form hydrogen cations or protons and carbon dioxide. The decomposition of organic compounds is carried out by electrogenic microorganisms or microbes capable of oxidizing the organic compounds and releasing electrons. An anode chamber creates an oxygen-free environment to facilitate anaerobic oxidation. Conversely, oxygen abundance is ensured in the cathode chamber to allow the reduction in oxygen molecules to generate water molecules. A membrane serves to impede oxygen from entering the anode chamber as well as allowing only protons to leave the anode chamber. However, a membrane may be absent depending on the design of the cells, which could be varied in a wide range to achieve desired efficiency, as stated by Slate et al. (2019).
Electrons flow from the anode to the cathode via an external circuit to complete the reduction process, generating electrical energy. With a larger scale of implementation, microbial fuel cells potentially produce sufficient electricity for various uses. The application of microbial fuel cells in the wastewater treatment process that often deals with sludge consisting of majorly organic compounds is highly anticipated as the technology uses wastewater to acquire renewable energy by utilizing the natural decomposition process of microbes.
Breaking down organic compounds in wastewater by microbes not only converts biomass energy to electrical energy but also decreases sludge production and the chemical oxygen demand of wastewater, which is important for wastewater treatment efficiency. Munoz-Cupa et al. (2021) reported that wastewater quality would affect the effectiveness of chemical oxygen demand reduction. The parameters considered for the suitability of a wastewater source for microbial fuel cell implementation include the availability of microbes, process duration, and concentration of phosphates, nitrates, oxidized nitrogen-containing compounds, or any other toxic compounds. Plus, the percentage of chemical oxygen demand can indicate electricity generation efficiency using a microbial fuel cell.
A microbial fuel cell can be installed in the soil where the organic substrates are the compounds in the soil. According to Shaikh et al. (2020), organic substrates in a plant microbial fuel cell are sourced from the rhizosphere, where plant roots release rhizodeposits or exudates from photosynthesis activities. Redox reaction in a plant microbial fuel cell is similar to that described previously. However, the anolyte is the deeper layer of soil lacking oxygen gas for anaerobic oxidation. A cathode is usually exposed to the atmosphere to allow contact with oxygen gases and is often called an air cathode. Microbes are readily present in soil and oxidize organic substrates into carbon dioxide and protons via the release of electrons. Figure 14 shows the schematic diagram of a simple plant microbial fuel cell.
Plant microbial fuel cell. A simple plant microbial fuel cell differs from a microbial fuel cell in terms of the medium used for ion navigation. Green plants enable effective conversion of carbon dioxide and carbon footprint reduction. By excluding electrolytes, the risk of toxicity and chemical pollution is reduced. The high availability of microbes in the soil is essential for achieving a better rate of electricity production. Key to maintaining microbial numbers and activities are the anode properties
Kabutey et al. (2019) believed that incorporating plants in the microbial fuel cell is advantageous in terms of the production cost due to the simplicity of construction that requires only electrodes installation in the soil and the high durability of the system. The system does not use potentially toxic or polluting chemicals and is reported with shallow environmental impacts. Nevertheless, the availability of microbes in the soil must be kept high to ensure high electricity productivity. The implementation of plant microbial fuel cells can be found in agricultural land and wastewater treatment plant where the source of nutrients in anolyte is sufficient for the metabolism of microbes.
Notably, the study of electrodes in microbial fuel cells has been the industry's focus for higher efficiency and cost reduction. The material selection criteria for the anode and cathode are different due to the different electrolyte properties of the two half-cells. Selection of anode material for the fuel cell that works in anolyte mainly comprising organic matters should consider the material's biocompatibility with microbes where antimicrobial activities shall be avoided to ensure effective oxidation of organic substrates.
As stated by Yaqoob et al. (2021a), the efficiency of organic substrate oxidation is significantly influenced by choice of suitable anode material, which directly impacts the overall productivity of a microbial fuel cell. Therefore, improving the anode is considered more crucial than improving the cathode. In accordance with Patwardhan et al. (2022), an anode in a microbial fuel cell shall be biocompatible, non-biodegradable, electrically conductive, porous, large in surface area, and have a lot of negative surface charges. Similarly, high porosity, electrical conductivity, and large surface area are preferred for the cathode material of microbial fuel cells. Finally, material cost, economic feasibility, stability, and durability are other considerations in selecting material for the anode and cathode of the cell.
Analysis of the effects of the abovementioned factors on the properties of biofilm formed on an anode surface is vital for the efficiency of electricity production. Besides, as stated by Yaqoob et al. (2021b), the removal efficiency of metallic contaminants such as cadmium relies on biofilm formation when implemented in wastewater treatment. They described a biofilm in a microbial fuel cell as the result of electrogenic microbes adhering to the anode surface, forming a layer of microbes. The stability of biofilm on the anode is higher than that cathode, indicated by the solid phase deposited on the anode surface compared to the slimy layer on the cathode surface. Due to biofilm's high stability, diffusion of electrons into the anode occurs with less hindrance than the cathode, realizing redox reactions that generate the current flow.
Palanisamy et al. (2019) stated that biocompatibility and surface area are especially important to achieve high microbe adhesion on the anode and efficiency of electron transfer and redox reaction. Hybrid materials often improve these factors due to anode surface modifications that allow more biofilm-anode surface interaction and create a larger reactive area with more microbes adhering to the anode surface.
Biocompatibility is measured mainly by the inhibiting effects on the growth of microbes in the anode compartment. The high growth of microbes is desired to ensure the desired size of the microbe community in the half-cell. Yaqoob et al. (2020) explained that significant toxicity and low corrosion resistance possessed by anode materials such as copper, silver, and gold have little biocompatibility for use in a microbial fuel cell, implying the adverse impacts of toxicity and corrosivity of anode material on the growth of microbes.
Despite metals being known for higher electrical conductivity, one of the wanted properties of the anode in a microbial fuel cell met, the implementation of metal electrodes is less widespread than carbon electrodes. Bensalah et al. (2021) stated that copper is the most electrically conductive of metallic anode materials. However, copper is subjected to severe corrosion issues, low biocompatibility, and poor stability, leading to poor performance in electricity production. Thus, in their study, the copper anode surface was coated with carbon nanofiber/polydimethylsiloxane composite to overcome the mentioned problems.
Anode surface properties such as surface area and hydrophilicity are prime influencers of the formation and characteristics of biofilm in a microbial fuel cell. Based on the study by Tripathi et al. (2022), low microbes' adhesion to the anode is often caused by the unavailability of active sites and the insufficiently large surface area of the anode. Moreover, they stated that the activation energy reduction for the decomposition process carried out by electrogenic microbes could not be realized without enhancing the electrode surface area. The absence of large reactive areas would eventually lead to inefficient electrical energy production in the microbial fuel cell. Besides, higher hydrophilicity was reported to positively affect the contact surface and microbe adhesion, offering the desired effectiveness and efficiency of the microbial fuel cell.
Surface modification using polymer coating is also investigated to improve metallic anode performance in a microbial fuel cell. As reported by Mwale et al. (2020), at least 96.24% of copper corrosion can be avoided by using polyaniline as the coating material of copper anode in a microbial fuel cell. Based on their research, the thin polyaniline layer on the metal surface creates a rough, porous, open, and highly branched microgranular structure. However, the poorly conductive coating layer and the passivated oxide layer between polyaniline and copper offer additional hindrances to electron transfer, causing lower power or current density.
Stability regards the ability to generate electricity at a constant rate. This is often checked with the profile of electricity production after steady-state conditions are accomplished. On the other hand, chemical and mechanical stability is also evaluated in terms of the performance of an anode material under different environmental conditions, such as pH and temperature. The durability of an anode material directly influences the practicality of the material in large-scale applications. For future long-term uses, chemical stability, mechanical stability, and biocompatibility are essential to achieve higher thermal stability, mechanical strength, and corrosion inhibition, respectively, according to Yaqoob et al. (2020). They also stated that the roughness of the anode surface could inhibit water molecules' adhesion to the anode surface and, thus, help preserve active sites for microbe biofilm growth.
Based on the same article, the high electrical conductivity of the anode facilitates and accelerates the flow of electrons emitted from the oxidation of organic substrates by microbes from the source to the anode. Without this property, a complete current flow from the anode to the cathode will not be formed due to the lack of attractive forces to pull electrons to the anode. Plus, the effect of bulk solution resistance on the electron flow would be reduced with high attractive forces given by the anode. In agreement with the previously made discussion, Ramya et al. (2022) also reported the significant effect of low electrical conductivity of biofilm-anode interface on electron flow. Table 9 summarizes the prime parameters in selecting the anode material of a microbial fuel cell based on the above-mentioned studies to provide an overview of the importance of anode material.
In the study of anode material for the operation of a microbial fuel cell, environmental conditions are manipulated to evaluate the suitability and practicality of the selected material in terms of the formerly discussed factors in Table 9. Environmental conditions can be altered in terms of temperature, pH, load of resistance, metal concentration, organic substrates, and hydraulic retention time. A study by Din et al. (2020) depicts the potential for power density improvement by optimizing pH, temperature, organic substrates, and resistance load. Tripathi et al. (2022) used polarization to achieve desired power density and microbe adhesion, enhancing fuel cell electricity production.
Almatouq et al. (2020) studied the effect of high acidity in the anode compartment of a microbial fuel cell on electricity production efficiency. Ideally, pH shall lie above 6.4 to avoid inhibiting microbe growth and biofilm formation. Additionally, they explained that the allowable length of hydraulic retention time depends on the species of microbes. Furthermore, oxygen diffusion into the anode compartment could be attributed to the prolonged retention time in the cathode compartment, which would cause organic substrates' degradation.
Carbon-based materials have conventionally been used as anodes for larger-scale production in microbial fuel cells. However, past application cases highlighted two significant problems carbon-based anodes: low electrical conductivity and high manufacturing cost. According to Bensalah et al. (2021), carbon-based anodes such as graphite and graphene are reported to have lower electrical conductivity, ranging from 104–105 S/m, which is 2 to 3 times lower than that of metals. Additionally, the production process for carbon-based anodes is generally more expensive. As a result of poor conductivity, resistivity to electron transfer and ohmic loss become more probable. On the other hand, Chen et al. (2019b) reported that hydrophobicity and low availability of reactive areas are implicit in carbon-based materials, hindering microbe attachment and the build-up of appropriate power density.
Carbon-based material is often used to produce a composite, which can be applied on metallic or carbon to fabricate an anode with desired properties for a microbial fuel cell. In accordance with Ramya et al. (2022), activated carbon and graphite are used in manufacturing the anode of a microbial fuel cell to achieve a meaningful scale of production. When incorporated with a metallic anode, the aim is to provide an additional layer of protection that helps decrease metals' degradation and corrosion.
In short, conventional carbon and metallic materials have inherent disadvantages that downgrade the overall electricity productivity of microbial fuel cells. Hence, surface modifications are a common practice in the industry to create better electrodes. For example, Saadi et al. (2020) showed that stainless steel coated with carbon nanofiber/polydimethylsiloxane composite has a lower corrosion rate in an acidic environment and higher biocompatibility in opposition to pure stainless steel. Even so, the layer of the coating increases the hydrophobicity of the anode and lengthens the start-up time of the fuel cell. Similarly, Masoudi et al. (2020) explored using acrylic-based graphite paint as a coating for stainless steel mesh to fabricate inexpensive electrodes with low toxicity, high power density, and improved biofilm thickness to other binding materials of graphite paint. Both articles used wastewater as the source of organic substrates.
Biochar is a much greener option than metallic compounds and carbon to manufacture anodes for a microbial fuel cell. The performance of using biochar in anode manufacturing has been studied intensively. Owing to the high electrical conductivity of metals, recent studies focus on using biochar to produce a composite material with metallic compounds, which aims to compensate for the flaws of metallic anodes. However, incorporating biochar in the fabrication of carbon-based anode also has considerable popularity due to the advantages offered. Notably, the researches significantly reduce large-scale microbial fuel cell application costs.
As discussed previously, biochar is fabricated from biomass via various thermal treatments to increase carbon content, and this process is generally known as carbonization. In many studies, biomass is sourced from agricultural wastes, which significantly contributes to the low cost of the electrode production process. Biomass is also acquired from different species of vegetation to be incorporated in the anode material of a microbial fuel cell.
Similarly, cathode material with a large specific area, electrical conductivity, stability, and durability is preferred in the application of microbial fuel cells. Nonetheless, the main goal in the cathode compartment is to increase the efficiency of the oxygen reduction reaction instead of maximizing the oxidation of organic substrates. According to Zhong et al. (2019), in the evaluation of cathode material, the focus is put on the oxygen reduction reaction as this is significant for the achievability of scaling up the implementation of a microbial fuel cell. This is because the power density and durability of the overall fuel cell rely on the reaction.
Besides cathode material physiochemical properties, the role of the catalyst has been studied intensively in the research field to improve oxygen reduction reactions in the air cathode compartment. Adding nanoparticles such as zinc/cobalt composites nanoparticles into the cathode compartment effectively reduces biofilm formation, as shown in the research by Yang et al. (2019). On the other hand, the biochar-derived catalyst has gained increasing interest for increasing the scalability of a microbial fuel cell. Table 10 lists recent studies that have utilized biochar made from various agricultural waste and vegetation as anodes, cathodes, and catalysts in microbial fuel cells.
The studies show a strong correlation between electrode surface topography and electrochemical performance. The graphitic structure is an important observation that indicates the effect of carbonization on biomass surface area. As discussed by Yang et al. (2018) and Hung et al. (2019), the graphitic structure of biochar positively impacts the electrical conductivity of the anode. With a more graphitic structure, biochar has identical electrical conductivity with activated coke. According to Chakraborty et al. (2020), graphitization which is often carried out at high temperatures, helps promote sp2 hybridization in carbon, improving electron mobility, leading to more efficient electron transfer and higher electrical conductivity. However, biochar's power density could be higher than activated carbon, as shown by the experimental study with cocklebur fruits.
Electrical conductivity is affected by the biofilm homogeneity and coverage on the anode surface. While the availability of active sites greatly depends on the specific surface area and porosity, the degree of coverage and homogeneity of biofilm on the anode surface is affected by material biocompatibility. The study on cocklebur fruits-derived biochar shows excellent biocompatibility of the anode material since the inner surface of pores on the anode was entirely covered with biofilm. Homogeneity of biofilm was observed with a similar thickness of biofilm present in the external anode surface and inner pores.
All anode materials derived from biochar exhibited excellent biocompatibility, which was evaluated in terms of toxicity and corrosion resistance, as well as the conduciveness of the environment provided for biofilm formation. Blending biochar with metal oxides has been found to avoid common issues associated with metallic anodes, including corrosion and toxicity, which can prohibit the growth and adhesion of microbes. Naveenkumar and Senthilkumar (2021) and Ramya et al. (2022) successfully demonstrated the deployment of biochar into metallic material to produce a cheap and effective anode for microbial fuel cells. The latter, however, also reported the catalytic effects of biochar on the redox reaction. Both studies show the high potential of copper to fabricate biochar-derived metallic anodes for use in microbial fuel cells. The addition of metal oxide nanoparticles into biochar-derived anode could potentially be an alternative to further increase the performance in electricity production as the presence of these nanoparticles was proven by Yaqoob et al. (2021c) to raise the electricity yield by promoting higher electron transfer, electrical conductivity, biocompatibility, durability, and stability. On the other hand, corrosion prevention contributes to the longer durability of the biochar-derived anode.
Clogging effects are mainly generated when the packed anode structure, addition of unsuitable material, and small pore size provide little space for biofilm formation and prohibit the mass transfer of organic substrates into the inner space of the electrode. Consequently, this could cause the death of microbes and the loss of electrochemical properties of the anode. Chen et al. (2019d) reported that an anode with a pore diameter below 200 \(\mathrm{\mu m}\) is prone to clogging, causing lower mass transfer and immobility of microbes into the pores. Conversely, Yang and Chen (2020) stated that penetration and growth of microbes are feasible in pores with a diameter of 10 \(\mathrm{\mu m}\). Nonetheless, the thickening of biofilm could increase the risk of future clogging. Plus, clogging could happen and cause failure to fully exploit all surface areas for biofilm formation due to small pores despite a large specific surface area.
The clogging effect shall be considered seriously to evaluate the long-term application of anode material in the electricity production of the microbial fuel cell. Therefore, researchers use large fibers, loose packing methods, and pore size enlargement to enhance clogging resistance. The loose packing method would have light and less dense biochar, advantageous for homogeneous and high-coverage biofilm generation, as discussed in the study on cocklebur fruits in Table 10. Nevertheless, Mohammadi Moradian et al. (2022) stated that the electrical conductivity of an anode is impeded by lightweight and low density.
In addition, an anode material may exhibit self-rechargeability due to its conducive environment and the adaptive evolution of microbes to live in a nutrient-limited environment. This may occur after a long usage period of a microbial fuel cell or when there is an inconsistent supply of organic substrates. This property was shown by the biochar-derived anode used in the experiment by Hung et al. (2019).
The range of power density of different biochar-derived anodes is wide, implying the significance of the choice of biomass source. Anode incorporated with almond shell and cocklebur fruit-derived biochar provided higher power density than carbon cloth and activated coke, respectively. The former achieved a maximum power density of 4346 mW/m2, which exceeded the power density of carbon cloth by 4094 mW/m2, implying the high potential of almond shell wastes to fabricate biochar-derived anode for electricity production in a microbial fuel cell.
Apart from electricity generation, the use of biochar-derived anode for higher wastewater remediation efficiency was investigated in the recent research by Chaijak et al. (2020) and Yaqoob et al. (2021c). Both types of research show the promising role of biochar-derived anodes in wastewater remediation. In many studies that describe the suitability of biochar as a microbial fuel cell anode fabricating material, anolyte was carefully selected to minimize the clogging effect. This poses limitations in the implementation of biochar-derived anodes due to the uncertainty of clogging when the fuel cell is supplied with actual wastewater.
Different activating agents were used to treat dried biomass prior to pyrolysis to improve the porous structure of the biochar-derived anode. Hung et al. (2019) and Kiminaitė et al. (2022) used potassium hydroxide solution to produce activated carbon from biomass. Based on the explanation, the alkaline solution would remove oxygen-containing functional groups from oxygenated compounds on the biochar surface to produce many pores during pyrolysis. Zinc oxide was used by Li et al. (2019) for biomass pre-treatment. In their study, biochar that was untreated with the chemical before pyrolysis has a very rough surface, causing insignificant or invisible macropores, which could raise the difficulty of mass transfer. They also reported the effect of optimum annealing temperature on the porous structure formed, as very high temperature could destroy the structure leading to pore fractures.
Conventionally studied cathode materials include platinum, stainless steel, graphite, carbon-based granular form, clothes, felt, and mesh. Biocompatibility of cathode material is less emphasized than anode material because, as Yang et al. (2019) explained, the mass transfer feasibility of oxygen and charged molecules is degraded by the biofilm formed on the cathode surface. Biofilm formation shall be limited in the cathode compartment because dense biofilm on the cathode surface would disturb the oxygen reduction reaction, thereby decreasing the electrical current production. This could potentially reduce biofilm coverage on the cathode surface. Nonetheless, porosity and specific surface area of the cathode are essential parameters in the performance of a microbial fuel cell, mainly in terms of the efficiency of the oxygen reduction reaction.
Nitrogen doping is a commonly found method to modify either cathode or catalyst to accelerate oxygen reduction reaction. Based on the result obtained in the research by Zhong et al. (2019), the graphitic structure of the surface of the biochar-derived cathode is crucial for better electrochemical properties in the cell. Not only that, the presence of pyridinic nitrogen and graphitic nitrogen was introduced as another key parameter in enhancing oxygen reduction reaction. Adding these two functional groups would offer more active sites for the reaction occurrence. Their result shows the superior performance in oxygen reduction possessed by nitrogen-doped biochar cathode when compared to commercially available platinum/carbon cathode. The larger the surface area or number of active sites, the larger the contact area between oxygen and electrons for reduction.
Similarly, for cathode catalysts, as stated by Li et al. (2018b), pyridinic nitrogen of cathode catalyst positively affects the rate of oxygen reduction reaction by effectively adsorbing oxygen and easing the process of electron transfer. The latter is helped by passing electrons from an anode to the cathode through the conjugated \(\uppi\) bond in the functional group. Their result presented the effect of a high pyridinic and graphitic nitrogen content, particularly in enlarging the specific surface area for electrochemical activity. Furthermore, the electrical conductivity and power production of systems with biochar catalysts exceeded those without catalysts. Still, more thorough investigations are needed on the actual mechanism of oxygen reduction reaction in the cathode compartment.
As previously mentioned, oxygen diffusion from the cathode to the anode shall be minimized to avoid disturbing an oxygen-lacking environment to facilitate the anaerobic decomposition of organic substrates at the anode. On the other hand, Chen et al. (2019d) stated that, in the cathode compartment, implementing a diffusion layer with high porosity for power density enhancement raises the risk of clogging. Thus, cathode clogging biofouling shall be avoided by ensuring efficient oxygen diffusion via back flushing.
Pyrolysis temperature plays a pivotal role in electricity production and wastewater remediation efficiency. In most of the studies reviewed, the effect of pyrolysis temperature on the biochar physiochemical properties was investigated. In agreement with Tomczyk et al. (2020), specific surface area, graphitized fraction, pH, and removal of volatile matters would be raised by implementing higher temperatures. In contrast, this could remove surface functional groups through bond breaking and rearrangement. Nevertheless, optimally high temperature shall be applied to avoid fracture of pores which potentially causes a reduction in electricity generation, as formerly discussed.
Studies have demonstrated the potential of biochar to act as an anode, cathode, and catalyst in a microbial fuel cell for electricity production, mainly due to the significant electrical conductivity and efficiency of redox reactions. However, for a larger scale of implementation, issues such as clogging effects and low scalability are due to inadequate power production and efficiency.
Biochar as a catalyst for biodiesel production
Biodiesel is an alternative liquid fuel to replace conventional diesel fuel as a transport fuel or for heat and power production. The common practice of biodiesel application in the transportation sector is to blend biofuel with petroleum fuel due to the high compatibility of biodiesel in the existing engine system. Biodiesel is derived from natural materials, mainly comprising vegetation and animal fats. Biodiesel production involves transesterification, which converts carboxylic acids of vegetable oils or animal fats into fatty acid esters in the presence of organic alcohols and catalysts. Glycerol is also produced as the only by-product in the transesterification of fats and oils. The reaction is illustrated in Fig. 15.
Transesterification of triglyceride into glycerol. Transesterification converts triglycerides to fatty acid methyl esters in the presence of methanol and catalyst with glycerol as a by-product. Fatty acid methyl esters can be used as biodiesel to be blended with conventional petrodiesel. Biochar can be used as a catalyst for a higher rate of biodiesel production. Biochar allows easier separation between the liquid product and the solid catalyst. Compared to homogeneous chemical catalysts, biochar typically exhibits high compatibility with raw materials, as well as recyclability, reusability, and lower environmental impacts
Biodiesel is a sustainable fuel choice since the feedstocks of biodiesel production are renewable and growable. Feedstocks' sustainability and renewability are vital for environment conservation and increasing national and global energy security. According to Zulqarnain et al. (2021), biodiesel has lower carbon and hydrocarbon emissions when combusted. When used as transportation fuel, the fuel provides a lubricating effect to a diesel engine and is highly compatible with direct usage in the engine. Another advantage mentioned is the higher flash point of biodiesel than petroleum diesel and pyrolysis oils. Therefore, the high flash point limits the formation of combustible and explosive vapor. Due to lower flammability, the use of biodiesel is considered safer when compared to conventional fuels.
Biodiesel is divided into five generations according to the types of feedstocks used in fuel synthesis. First-generation biodiesel, produced from edible oil-crops such as sunflower, soybean, rapeseed, palm oil, and coconut, possesses high similarity with petroleum diesel. Moreover, the first-generation biodiesel production process is relatively more straightforward, leading to high popularity among different generations of biodiesel. The second generation of biodiesel refers to those that use non-edible crops as feedstocks. Non-edible crops offer zero impacts on global food security as well as a higher yield of biodiesel, as stated by Shaah et al. (2021). Still, Zulqarnain et al. (2021) explained that the expansion of non-edible crop-derived biodiesel production could bring land and water issues besides the high cost of production, which become the primary resistance in the production of second-generation biodiesel.
Seaweeds are classified as third- or fourth-generation feedstocks (Farghali et al. 2023a). Thus, biodiesel produced from wastes and algae is referred to as third-generation and fourth-generation biodiesel, respectively. Zulqarnain et al. (2021) believed that household, agricultural, and animal fat waste are the ideal biodiesel production feedstock. This is because of the high availability and accessibility of waste. Problems encountered in the production of first-generation and second-generation biodiesel, including food insecurity, deforestation, water issues, and high cost, can be avoided by the utilization of wastes in producing biodiesel.
Fourth-generation biodiesel is produced from algae. Referring to Bošnjakovi´c and Sinaga (2020), biodiesel production with algae has greater productivity due to the shorter cycle of algae growing in the range of one to ten days. However, the inconsistency is found in the production cost, causing inconstancy in the fourth-generation biodiesel price. The high price of biodiesel on the market is also attributed to the less developed technology and low quantity per harvest to reduce the cost of production. For example, following them, the high power consumption of paddlewheels contributes to the production cost despite the high harvesting efficiency offered by the open-pond farming system. Considerably large energy intensity and production cost are the two prime challenges faced in the fourth-generation biodiesel industry.
Lastly, fifth-generation biodiesel is derived from genetically engineered crops that undergo tuning and alteration of genes to fulfill specific properties that could help in advancing the efficiency in terms of costs and process to produce biodiesel. One example provided by Chaturvedi et al. (2020) is improving the content of lipids or triacylglycerols in the biomass, particularly microalgae, through gene coding of enzymes involved in the conversion of intermediate products in the lipid synthesis pathway. Conversely, lipid content can be enhanced by removing enzymes that impede the lipid synthesis pathway. Both methods aim to promote a high rate of lipid synthesis. The synthesis pathway of other natural components, including starch and lipolysis, that consumes the materials of lipid synthesis is limited by hindering the associated enzymes from allowing higher availability of materials for lipid generation.
The application of biomass in biodiesel production has been studied intensively and applied for a long time. While the advantages of using biomass to synthesize biodiesel are widely known, the role of biochar as a green catalyst in biodiesel production has gained increasing interest. Catalysts used in biodiesel production can be classified into two major groups: homogeneous and heterogeneous. Biomass-derived catalyst often appears solid, allowing easier separation from the liquid biofuel.
According to Changmai et al. (2020), heterogeneous catalysts, especially those derived from biomass, experience increasing popularity due to several offered advantages. Homogeneous catalysts, which could be either strong bases or strong acids, are advantageous due to high availability and accessibility as well as good catalytic effect. Nevertheless, lower yield due to the formation of by-products, incompatibility of the catalysts with some fats or oils, and low sustainability of homogeneous inorganic catalysts are the major drawbacks of long-term application. In contrast, heterogeneous catalysts are known for their high sustainability, as they are recyclable and reusable and have low toxicity, corrosive effects, and energy demands.
Recent studies focus on using bone and shell wastes and vegetation to synthesize biochar-derived catalysts for biodiesel production. Various biomass sources are used as the feedstock for the transesterification process. Table 11 summarizes the result of the recent studies. Overall, the maximum biodiesel yield is between 80% and 98%, except for the experimental study by Anto et al. (2019), which used microalgae as transesterification feedstock and dried leaves as raw material for catalyst production.
The procedures of producing biochar from animal bone and shell wastes differ from that of vegetation. While biochar production from vegetation primarily uses pyrolysis at a temperature between 350 and 700 °C, a higher temperature range is implemented for the thermal treatment of bones and shells to produce basic biochar catalysts, often referred to as calcination.
Bones and shells receive growing interest in studying biodiesel production catalysts due to the high content of calcium-containing compounds that could act as basic catalysts. As reported by Tan et al. (2019), Yaşar (2019), Khan et al. (2020), Lin et al. (2020), and Rahman et al. (2021), the calcination temperature is between 600 and 1000 °C. In a study by Khan et al. (2020) on the calcination temperature range of 600–1000 °C, the highest catalytic effect was observed at 900 °C. Although a calcination temperature above 900 °C is recommended, further optimization is necessary to obtain the highest possible calcium oxide content, which serves as a natural and effective basic catalyst.
The maximum yield of biodiesel using bone- and shell-derived catalysts generally falls in the 87–97% range. Yaşar (2019) compared the performance of eggshell and calcinated eggshells in producing biodiesel from rapeseed oil. Eggshells naturally contain calcium carbonate, which can be transformed into calcium oxide through a calcination process at an elevated temperature of around 950 °C. Calcium oxide is a highly effective transesterification catalyst. In a study on using egg shells as a feedstock for catalyzing biomass transesterification, researchers found small performance differences between eggshells and calcinated egg shells, with 95.12% and 96.81% yields, respectively. This indicates the high potential of using eggshells as a catalyst for transesterification. Similarly, Rahman et al. (2021) reported a maximum biodiesel yield of 93.27% in the calcinated eggshell-catalyzed cooking oil transesterification.
Tan et al. (2019) used chicken bones and fish bones as the feedstock of catalysts and achieved a maximum biodiesel yield of 89.5%. Khan et al. (2020) and Lin et al. (2020) calcinated ostrich bones and oyster shells, respectively, to produce a basic catalyst for cooking oil transesterification. The highest biodiesel yields were reported as 90.56% and 87.3%.
The sulfonation of biochar can produce acidic biochar catalysts. Sulfonation is the acid treatment using the concentrated sulfuric acid solution at elevated temperatures. The purpose of sulfonation is to add acidic active sites on the surface of the biochar. Other approaches for the same purpose are copolymerization and treatment with a weak acid solution, but treatment with sulfuric acid remains the most popular way to sulfonate biochar.
Behera et al. (2020) treated sugarcane bagasse, corncob, coconut shell, and peanut shell with 10 mL of 98% sulfuric acid solution while heating at the boiling point of water for 1 h. Based on their research, the natural properties of biomass feedstocks and pyrolysis temperature significantly impact the sulfonic acid density. A negative correlation between pyrolysis temperature and sulfonic acid density was reported in their study. High pyrolysis temperature favors complete combustion and aromatization, leading to a high degree of crosslink formation and polymerization. This impedes the generation of defective polycyclic aromatic sheets used to retain the acidic active sites, as Konwar et al. (2019) stated.
Surface acidic active sites are indicated by the sulfo group with a chemical formula of –SO3H. The effects of surface acidification include increased density of the sulfo group and greater surface area of biochar due to bigger pore size, as explained by Cheng and Li (2018). Increased surface area and active sites enhance biodiesel production's reaction rate and efficiency. In the study by Mohamed et al. (2020), the maximum biodiesel yield was 90.37% when the transesterification was conducted with a rice straw-derived catalyst of 10% by weight at 70 °C for 6 h. Behera et al. (2020) reported 91.04% of the maximum biodiesel yield from microalgae. The optimum conditions were a catalyst dose of 5% by weight, transesterification temperature of 65 °C, and reaction time of 4 h. On the other hand, the highest biodiesel yield obtained by Taufiq et al. (2020) was lower, recorded as 89.71% with a reaction temperature of 60 °C and 3% of Sengon sawdust-derived catalyst.
However, unsulfonated biochar-derived catalysts could provide biodiesel yield as high as sulfonated biochar. Zhao et al. (2018), Basumatary et al. (2021), Kim et al. (2021), Sahu (2021), and Daimary et al. (2022) reported the maximum biodiesel yield exceeding 95% by using pomelo peel, Kesseru plant, peanut waste, rice straw, and potato peel as the raw materials for the synthesis of biochar-derived catalyst, respectively. The maximum yield was recorded as 98%, 97.75%, 95.4%, 97.3%, and 97.5%. Interestingly, the quantity of biodiesel produced from the transesterification process of cooking oil, catalyzed by unsulfonated rice straw biochar, was higher than sulfonated by 6.93%, referring to Mohamed et al. (2020) and Sahu (2021). Sulfonation is necessary for treating biochar that has significant disadvantages in terms of pore size and availability of the active site. The maximum biodiesel yield from transesterification of brown marine microalgae is the lowest, only 32.8% and 59.06% for Isochrysis sp. and Phaeodactylum tricornutum, respectively, as reported by Anto et al. (2019).
The operating conditions, such as reaction temperature, time, feedstock ratio, and catalyst dosage, greatly influence the transesterification yield. The availability of organic acids and oil is crucial for high yield. It is important to explore the optimum catalyst dosage, as the availability of raw materials can often limit the reaction yield. Similarly, the optimum reaction time should be determined as prolonged reaction times beyond the saturation point do not necessarily improve the biodiesel yield. By implementing optimal reaction time and catalyst dosage, both capital and operating costs can be reduced.
Reaction temperature plays a pivotal role in manipulating the reaction kinetics and equilibrium. Temperature elevation could shift the equilibrium toward the direction favoring transesterification and improving the reaction rate. However, if the temperature surpasses the optimal range, the reaction yield is negatively impacted due to the heightened saponification rate. Typically, the ideal transesterification temperature falls between 60 °C and 65 °C.
Recent studies also show the high reusability of biomass-derived catalysts for the transesterification of biomass. The biodiesel yield remained above 80% after eight cycles of transesterification using pomelo peel and rice straw biochar-derived catalysts, reported by Zhao et al. (2018) and Mohamed et al. (2020), respectively. Conversely, Sahu (2021) reported a decrease of 44% after nine cycles of transesterification catalyzed by rice straw biochar.
Referring to Tan et al. (2019), Behera et al. (2020), and Daimary et al. (2022), biodiesel yield reduction was 35%, 15.06%, and 8.5%, respectively, after five cycles, showing the superiority of potato peel as sustainable and cheap feedstock for biochar-derived catalyst production. Yaşar (2019) reported a decline of 2.32% and 2.1% for calcinated and raw egg shells, respectively, while Basumatary et al. (2021) reported that the yield was 7.53% lower after three cycles of transesterification. Khan et al. (2020) found that biodiesel yield using calcinated ostrich bones as a catalyst remained above 80% after four cycles.
The decline of biodiesel yield after several cycles is mainly attributed to the loss of active sites on the catalyst's surface. At one point during several cycles, the yield drops drastically, indicating the deactivation of the biochar catalyst. Based on the explanation by Tan et al. (2019), the catalyst particle size increases due to the adsorption of oil and water as well as impurities. This hinders the catalysis process by reducing the exposed surface area per unit mass and the number of active sites. Similarly, Sahu (2021) stated that the adsorption of glycerol on the catalyst surface and the degradation of sodium ions contribute to deactivation. The availability of different functional groups after several cycles of transesterification ensures the reusability of a catalyst. As Behera et al. (2020) explained, besides sulfo groups, the desired functional groups include carbonyl and carboxyl. Past studies show that carbonyl and carboxyl groups are useful in removing heavy metals in the samples.
Although biochar is a promising material for synthesizing cheap, sustainable, environmentally friendly, and highly effective catalysts for biodiesel production, the main drawback is similar to other implementations of biochar, which is the high energy demand and cost of the biochar preparation process. Still, biochar catalysts are advantageous by providing another approach to utilizing food and agricultural wastes and enhancing the greenness and efficiency of biodiesel production.
Biochar as a catalyst in biohydrogen production
Hydrogen gas emerges as an alternative electrical energy source to replace fossil fuel-burning power production. This energy source poses minimal polluting effects in energy production as the only by-product is water. Hydrogen is used as electric vehicle transport fuel and raw materials in the production industry, heat, and power generation. Different feedstocks of hydrogen synthesis affect the sustainability, capital investment, and operating cost of the production plant due to the dissimilarity in the processing scheme.
Referring to Gemechu and Kumar (2021) and Ajanovic et al. (2022), the production schemes of hydrogen are color-coded based on the type of feedstocks and the production process. Gray hydrogen production involves steam reforming of natural gas, mainly methane, and coal gasification, which releases carbon dioxide as the main by-product. Despite the significant environmental impacts, gray hydrogen production by steam methane reforming remains a major part of the industry due to the maturity of the technology and availability of feedstocks. Blue hydrogen production differs from gray hydrogen production as the former includes carbon capturing and storing. Turquoise hydrogen is synthesized from fossil fuels through a pyrolysis process comprising thermal decomposition, plasma decomposition, and catalytic decomposition. Solid carbon is emitted as the by-product of turquoise hydrogen production.
Compared to thermal treatment, electrolysis is a more attractive option due to lower environmental impacts. To generate green hydrogen, water undergoes electrolysis with a renewable electricity supply. Deployment of renewable electricity realizes zero carbon emissions. Nuclear energy is also implemented to produce pink hydrogen, while grid electricity, generated from both renewable and fossil energy, produces yellow hydrogen. Feedstocks of hydrogen production are classified into fossil fuel, biomass, and water. Hydrogen from biomass is often called green hydrogen due to its carbon neutrality.
At the current state, the role of biochar in the production of biohydrogen via water splitting, methane conversion, and anaerobic digestion has been explored in recent studies. In the following sections, a review focuses on the potential application of biochar in water splitting, methane conversion, and anaerobic digestion.
Biochar role in water splitting
Conventional water splitting to produce biochar through electrolysis consumes electrical energy for redox reaction as the following:
Anode: 2H2 \(\rightarrow\)O2 + 4H++ 4e−
Cathode: 4H++ 4e− \(\rightarrow\) 2H2.
Overall: 2H2 \(\rightarrow\)2H2 + O2.
Different materials have been used as an electrocatalyst in water electrolysis to reduce the reaction activation energy and biohydrogen production cost. Electrocatalysts can be carbonaceous and metallic compounds or a combination of the two materials that appear in different forms. The performance of biochar in electrocatalytic water splitting has been discussed in recent research. Biochar with excellent electrochemical properties is introduced as a greener option to replace conventional metallic and carbonaceous catalysts.
Solar-to-hydrogen conversion system, a solar energy-based water electrolysis system, can be divided into photocatalytic and photoelectrocatalytic types. According to Mohamed (2022), photo-electrocatalytic and photocatalytic water splitting is advantageous due to the omittance of wires and external electronics, close-loop cycles, incorporation of renewable power sources, and reduced cost from cheap semiconductor materials as adsorber and energy storage. As Bhakta et al. (2022) and Mohamed (2022) explained, photons from solar energy activate photoanode and photocathode. In a photo-electrocatalytic system, a photoanode requires a constant supply of bias potential to induce the movement of photoelectrons from one cell to the other. Photogenerated electrons flow to the cathode compartment to combine with protons to synthesize hydrogen gas. In this process, holes are generated on the anode surface. The holes act as an oxidizing agent of water to form oxygen gas.
Mohamed (2022) stated that, in photo-electrocatalytic water splitting, solar energy is directly transformed into chemical energy. Therefore, the potential of biochar as a catalyst or a support material in photo-electrocatalytic water splitting needs further investigation. This could be beneficial in improving the performance of the water-splitting system, reducing cost, and promoting the sustainability of biohydrogen production.
In photocatalytic water splitting, the deployment of biochar can be found in the form of a combination with conventional semiconductors. According to Bhakta et al. (2022), a common problem in photocatalytic water splitting is photocatalyst deactivation caused by the fusion of photoelectrons and holes. To address this issue, organic compounds called hole scavengers are often added to eliminate holes on the catalyst surface. Biochar is also incorporated into hydrogen production via assisted water electrolysis as a sacrificial electrode. In assisted water electrolysis, energy demanding oxygen evolution reaction is substituted with an oxidation process that uses various materials to reduce the energy requirement. Carbon materials are widely studied due to their high potential for cheap and energy-efficient water electrolysis. Unlike oxygen evolution reactions in normal water splitting, carbon dioxide is emitted as the by-product in carbon-assisted water electrolysis.
Referring to Chen et al. (2019c), carbon-assisted water electrolysis is preferred because of lower power consumption, attributed to the smaller standard potential required, which is 0.21 V, as compared to normal water splitting with a standard potential of 1.23 V. The followings are the half equations and overall equations representing the above-mentioned electrolysis scheme:
Anode: C + 2H2O \(\rightarrow\)CO2 + 4H++ 4e−
Cathode: 4H+ + 4e−\(\rightarrow\)2H2.
Overall: C + 2H2O\(\rightarrow\)2H2 + CO2.
With the lower energy demand of carbon-assisted water splitting, biochar is a promising material to replace conventional carbon materials to achieve carbon–neutral water electrolysis. This is possible because of the excellent electrochemical properties of biochar.
The schematic diagram of electrocatalytic water splitting, photocatalytic electrolysis water splitting, photo-electrocatalytic water splitting, and carbon/biochar-assisted water electrolysis is presented in Fig. 16. Additionally, Table 12 summarizes various studies that have explored the potential of biochar in enhancing biohydrogen production via water splitting. Results from these studies suggest that biochar holds significant promise in improving biohydrogen production through experiments with biochar-assisted electrolysis, electrocatalytic splitting, and photocatalytic splitting.
Biohydrogen production via water splitting. Due to its excellent electrochemical properties, biochar acts as the catalyst in four setups. Biochar-assisted water splitting produces carbon dioxide, the by-product absent in the other setups. Biochar-assisted water splitting has lower energy consumption and higher efficiency than the standard water electrolysis process. Biochar properties, including the surface functional group, porosity, and porous structure, impact ion transportation and electrolysis efficiency
In assisted water electrolysis, biochar can be applied in pure form or combined with metallic materials to form composite electrodes. Chen et al. (2019c) used spent biochar from wastewater treatment to generate biohydrogen. The result shows the excellent performance of biochar-assisted water splitting in terms of energy requirement compared to the standard water electrolysis and oxygen evolution reaction. This is indicated by the very low overpotential of the biochar-assisted system, recorded as 0.5 V. Their study also shows the importance of using optimum pyrolysis temperature to achieve higher current density by maximizing porosity and graphitization while conserving pore structure.
Significantly, Chen et al. (2019c) restored biochar oxidation activity through heat treatment, enabling its reuse for biohydrogen production via biochar-assisted water electrolysis. They recommended using carbon dioxide from electrolysis for biochar fabrication via carbon dioxide gasification, which they found to be a more efficient production pathway than heat treatment. This approach promotes sustainability and reduces carbon emissions in biohydrogen production.
Naturally, mediators such as chloride ions were said to be effective in further improving biochar oxidation reaction rate, as Chen et al. (2019c) mentioned. Ying et al. (2021) added ferrous(II) cations, ferrous(III) cations, nitrite cations, and nitrate cations as mediators in rice husk-derived biochar-assisted water electrolysis. Ferrous(II) and nitrite ions exhibit high biochar oxidation activity, lowering the onset potential. However, the increase in oxygen evolution rate with ferrous(II)/ferrous(III) ions is greater than that with nitrite/nitrate cation, which is inhibited by a side reaction involving the nitrite ions. The side reaction raised the onset potential after 40 min of use. However, Ying et al. (2021) suggested reducing the sulfuric acid concentration to restrict the side reaction.
This is supported by the experimental result of Du et al. (2022) and Ying et al. (2022), who correlated the acidity of electrolytes and the diffusivity of ions. Based on the experiment result by Du et al. (2022), reducing acidity yielded a higher diffusion coefficient in the anode compartment and improved the rate of the biochar oxidation reaction. This property was only observed in the biochar-assisted system, not in normal oxygen evolution reactions. Conversely, neutrality and acidity brought about a higher adsorption rate of sulfate anions from the electrolyte, causing the variation in electrode surface charge distribution, electric double-layer composition, and reduced active sites. Ying et al. (2022) explained that increased viscosity of the acid solution at higher concentrations makes ion diffusion more difficult. Also, a higher concentration of the electrolyte hinders the uniform distribution of ions, owing to the reduced adsorption ability of biochar.
Ying et al. (2022) compared the performance of carbon black, coal, and biochar produced from pyrolysis and hydrothermal treatment in assisted electrolysis. Carbon-assisted water electrolysis reduced energy consumption compared to sulfuric acid solution electrolysis across all materials tested. Pyrolysis-produced biochar exhibited the most significant current density among different carbonaceous materials. The pyrolysis temperature is crucial in protecting pore structure, improving porosity, increasing active sites, and facilitating more accessible ion transportation, significantly impacting the electrolysis rate. Similarly, Ying et al. (2022) found that acid treatments effectively improve the abovementioned elements. Specifically, hydrochloric acid treatment exposes surface functional groups, providing a higher rate of biochar oxidation reaction, while hydrofluoric acid removes silicates that often act as biochar oxidation reaction inhibitors.
Overpotential and current density are also the main indicators used to study the effect of biochar catalysts in electrocatalytic water splitting. Biochar exhibited excellent catalytic activity, shown by the improved hydrogen evolution reaction rate measured in terms of overpotential and current density. Biochar was applied in different forms by Humagain et al. (2018), Deng et al. (2021), Yang et al. (2021), and Chen et al. (2022b). When compared between the studies, with suitable preparation and treatment of biochar, metallic materials can be used to further increase the catalytic activity of biochar.
Studies have demonstrated that similar to assisted electrolysis, the rate of water electrocatalysis is influenced by various biochar properties, including surface functional groups, porosity, and porous structure, which can be modified via acidic or basic treatment, pyrolysis temperature, and the addition of metallic compounds. However, another important consideration of catalysts is their stability and durability. Therefore, stability and durability are to be evaluated by conducting multiple runs of electrocatalysis and observing the loss of current density or deactivation after a long operation.
Based on the experiment results of recent studies, biochar-derived catalysts exhibited high durability and stability for long-term use. As reported by Humagain et al. (2018), who fabricated birch tree wood residue-derived activated carbon doped with ammonium molybdate, after 100 h of operation, the potential of the cell was maintained at 35 mV and 60 mV with the constant current density of − 10 mA/cm2 and − 100 mA/cm2, respectively. They also stated that the small dissolution of the molybdate atom reflects the stability and durability of the fabricated catalyst. Yang et al. (2021) used watermelon peel biochar doped with cobalt for the electrocatalysis of water. They reported that for 20 h of use, a small increase in overpotential, recorded as 0.06 V, was noticed at a constant current density of 10 mA/cm2. On the other hand, Chen et al. (2022b) reported a 5% loss of current density and a 2 mV increase in overpotential after one day of running the electrolysis process with acid-treated waste rice husk biochar as the electrocatalyst.
The stability of photocatalysts is also studied intensively in the research focusing on biochar-derived photocatalysts. Xu et al. (2022) attributed the loss of photocatalytic activity to photocorrosion. Photocorrosion can be caused by an oxidation process that produces holes on the catalyst surface. While Chen et al. (2019a) and Xu et al. (2022) reported the superiority of biochar-derived photocatalysts in photocatalytic water splitting, photogenerated holes can contribute to the deactivation of the catalyst in the long run. Thus, a hole scavenger such as lactic acid used by Xu et al. (2022) is essential for limiting the combination reaction between the holes and the photo-electrons. The catalyst's exposure to the light source is influenced by its properties, such as carbon composition, and is another significant factor., as Xu et al. (2022) reported.
In a nutshell, biochar can be applied in different types of water-splitting processes and provide a high rate of biohydrogen production owing to its excellent catalytic and oxidation activity. Problems with implementing biochar-derived materials in water splitting were addressed, and solutions are proposed in recent studies. However, the process's expensive cost and scaling up remain the biggest challenge to promoting widespread implementation.
Biochar role in methane to hydrogen
There are three pathways for converting methane gas to hydrogen gas: steam methane reforming, partial oxidation of methane, and dry reforming of methane. Referring to Patel et al. (2020), the reactions that happen in each pathway are as follows:
1. Steam reforming: CH4 + H2 \(\rightarrow\) CO + 3H2 \(\Delta {\mathrm{H}}_{25}\mathrm{^\circ{\rm C} }\) = 206 kJ/mol.
2. Partial oxidation: CH4 + ½ O2 \(\rightarrow\) CO + 2H2 \(\Delta {\mathrm{H}}_{25}\mathrm{^\circ{\rm C} }\) = –23 kJ/mol.
3. Dry reforming: CH4 + CO2\(\Delta {\mathrm{H}}_{25}\mathrm{^\circ{\rm C} }\) CO + 2H2 \(\Delta {\mathrm{H}}_{25}\mathrm{^\circ{\rm C} }\) = 247 kJ/mol.
Steam reforming, partial oxidation, and dry reforming feedstocks are steam, oxygen, and carbon dioxide supply, respectively. As previously mentioned, gray hydrogen production dominates the industry, while steam reforming methane remains the most implemented method to produce gray hydrogen. Methane decomposition via steam reforming is energy intensive due to the high energy requirement of reaction activation. The reaction temperature is approximately 1000 °C, causing large energy demand for reactant preparation. Due to the considerably large energy consumption of sensible heat and reaction heat, gray hydrogen production via steam reforming is highly environmentally polluting, with large carbon emissions.
Catalytic methane decomposition can reduce the energy demand and carbon emission compared to steam reforming and dry reforming, as it lowers the activation energy requirement and enhances the reaction kinetics. The specific energy consumption of catalytic decomposition is reported to be 36.70% and 30.61% of the energy requirements for steam reforming and dry reforming, respectively. The reaction for catalytic methane decomposition is as follows:
CH4C + H2 \(\Delta {\mathrm{H}}_{25}\mathrm{^\circ{\rm C} }\) = 75.6 kJ/mol
The reaction temperature of catalytic methane decomposition lies in the range of 800–950 °C.
Metallic materials are used as conventional catalysts in the catalytic decomposition of methane. Metallic materials are preferred because of two hydrogen yield-promoting factors, including the excellent catalytic activity and the enhanced morphologies of nano-carbon deposits, as Patel et al. (2020) explained. Nonetheless, they mentioned several disadvantages of metallic materials: high cost, deactivation caused by carbon deposits on the catalyst surface, difficult catalyst-carbon separation, and low catalyst regeneration. Carbon materials are studied intensively to replace or combine with metals for catalytic methane decomposition as the materials overcome the flaws of metallic catalysts. However, the performance of carbon-based catalysts is often lower. In some cases, metallic and carbon materials are implemented simultaneously by fabricating composite materials to increase the process and cost efficiency.
While the use of activated carbon in catalytic methane decomposition is widespread, biochar has gained increasing interest in lowering the carbon footprint of methane conversion to hydrogen. Table 13 shows the results of recent studies that focus on using biochar in catalytic methane decomposition and dry methane reforming.
Conversion of methane varies in a wide range depending on the catalyst materials and biochar compositions. In the study by Harun et al. (2020), Douglas fir-derived biochar was prepared and used to produce potassium hydroxide-activated and heat-treated biochar. The catalysts were compared with commercial activated carbon and zeolite Socony Mobil-5 doped with 3% ruthenium. Ruthenium-activated carbon achieved the highest methane conversion, recorded as 73%. The methane conversion of potassium hydroxide-activated biochar was 69%. Methane conversion of heat-treated biochar and zeolite Socony Mobil-5, doped with ruthenium, had comparable methane conversion, recorded as 41% with 40%, respectively.
Compared to the surface area, activated biochar had a significantly larger area than ruthenium-activated carbon. Importantly, activated biochar still possesses the largest surface area, although the surface area shrunk by 41.86% and 77.09% after 8 h and 60 h of reaction, respectively. This property is advantageous to prevent the deactivation of a catalyst as carbon produced from methane decomposition easily clogs the pores, primarily micropores, leading to decreased active sites for catalytic activity.
In the experiment by Patel et al. (2020), biosolids-derived catalysts without acid or base activation show excellent catalytic activity compared to the biomass-derived activated carbon in past studies, including coconut shells, hardwood, vegetable, and olive stones. Maximum conversion of methane at the initial stage was recorded as 65% and 72% for biochar and sodium hydroxide-activated biochar, respectively. Higher methane conversion of activated biochar may be attributed to greater surface area and smaller pore volume.
Patel et al. (2020) also reported the significance of catalyst surface oxygen-containing groups in accelerating methane conversion to hydrogen. Oxygen-containing groups, primarily carboxylic groups, were studied in their research. The experiment result shows that carboxylic groups become thermally unstable and decompose at high temperatures. This releases carbon dioxide and carbon monoxide, providing more active sites on the catalyst's surface. The authors also reported surface area reduction by approximately 90% after 6 h due to pore clogging by the produced carbon.
Zhao et al. (2022b) used Enteromorpha prolifera to produce biochar and phosphoric acid-activated biochar. Their result is similar to the literature discussed, where acid or base treatment effectively increases the surface area. Importantly, their result showed reduced methane conversion when excessive phosphoric acid was used to treat the biochar. Acid and base treatment increase the surface area by improving the porosity of the surface through the production of volatile compounds when reacts with biochar. Excessive addition of acid or base could lead to high porosity and destruction of pore structure. The authors also explained that decreased conversion at excessive acid treatment could result from generating insulation on the catalyst surface, impeding the specific surface area enhancement.
Kundu et al. (2021) examined using microcrystalline cellulose-derived biochar to produce carbon-encapsulated iron nanoparticles. Methane conversion of the fabricated catalyst is the highest, recorded at 95.7%, compared to the formerly discussed biochar catalysts. Based on their study, an increase in iron composition could lead to the depletion of catalyst surface area. In contrast, carbon deposits become the main inhibitor of catalytic activity at low iron loading.
The catalytic decomposition of methane is temperature dependent, as depicted in the study by Zhao et al. (2022b). Since catalytic methane decomposition is endothermic, higher temperature thermodynamically enhances the reaction yield. Studies also show a negative relation between methane composition in feed and methane conversion.
Li et al. (2018a) studied the role of biochar-derived catalysts modified with metal nanoparticles in dry methane reforming. The maximum average conversion of methane was reported as 76.8% and 86.78% for cotton stalk biochar-derived catalysts and that modified with nickel nanoparticles, respectively. Their result implies the significance of adding suitable alkaline earth metal nanoparticles in biochar catalyst for higher methane conversion. Carbon dioxide conversion is another prime indicator of catalyst effectiveness in dry reforming. Alkaline metals are found to help improve carbon dioxide conversion. Another factor affecting the reaction yield is the particle size of the catalyst.
Dry reforming does not produce solid carbon as the by-product. Still, carbon is generated from carbon gasification that consumes carbon from the catalyst. Therefore, carbon deposit clogging the catalyst surface pores hinders the methane conversion in the dry reforming process. The authors reported that the constant and low conversion at the experiment's final stage was attributed to methane's thermal decomposition, implying that the deactivation of the catalyst occurred for a long time.
Overall, acid and base treatment are essential to achieve higher methane conversion. Optimum acid/base and temperature values shall be explored for specific types of biochar-derived catalysts. Deployment of a small quantity of metal in biochar-derived catalyst has a considerably high catalytic effect and hence is considered promising to achieve high methane conversion. Notably, the common problem in biochar-catalyzed methane decomposition is pore clogging due to carbon deposits on the catalyst surface.
While biochar-derived catalyst realizes cheaper and less energy-intensive production of hydrogen from methane, the reported methane conversions in the pieces of literature are generally below 72% without incorporating metal. Studies should focus on the recyclability and efficiency of catalysts to ensure the sustainability of catalytic methane reforming.
Biochar role in hydrogen production from anaerobic digestion
Anaerobic digestion is a decomposition process by different microorganisms to decompose large organic matter into simpler compounds. As the process produces hydrogen gas as an intermediate product, the potential of anaerobic digestion to produce biohydrogen as a mechanism of waste-to-energy has been investigated. This section focuses on the role of biochar in improving biohydrogen yield and production rate in anaerobic digestion.
Application of anaerobic digestion is widespread in the wastewater treatment plant and is often conducted after aerobic digestion of wastewater. In the wastewater treatment plant, anaerobic digestion is denitrification, which converts nitrates to nitrogen gas. Anaerobic digestion removes other organics, including carbohydrates, proteins, and fats. This is attributed to the presence of various kinds of microorganisms living on different substances in solid wastes and wastewater. Figure 17 illustrates the four stages of anaerobic digestion and the downstream process.
Biohydrogen production via anaerobic digestion. Microbial activities are crucial in decomposing organic substrates, including solid wastes and wastewater. The process involves hydrolysis of complex organic matter, followed by acidogenesis, which produces volatile fatty acids, acetic acid, hydrogen, and carbon dioxide. Acetogenesis converts volatile fatty acids into hydrogen, carbon dioxide, and acetic acid. The methanogenesis process, which consumes hydrogen for methane synthesis, must be suppressed to achieve a high biohydrogen production rate. This can be achieved by manipulating the operating conditions, including pH, temperature, carbon-to-nitrogen ratio, and retention time, to reduce the methanogenesis rate
Referring to Muthudineshkumar and Anand (2019), Janajreh et al. (2019), and Khalid et al. (2021), anaerobic digestion consists of four stages taking place sequentially to convert large organic matter to methane, inorganic carbon, and hydrogen. The process starts with the hydrolysis of complex organic matter. At this stage, carbohydrates, fats, and proteins are broken down by fermentative bacteria to form soluble organic molecules such as sugars, fatty acids, peptides, and amino acids. The process is continued with acidogenesis, where soluble organic compounds are converted to volatile fatty acids such as propionic acid and butyric acid by fermentative bacteria. A small concentration of acetic acids, hydrogen, and carbon dioxide is synthesized simultaneously from ethanol and butanoic acid-producing fermentation at this stage.
The amount of volatile fatty acids produced in the acidogenesis stage directly impacts the production of hydrogen gas in the subsequent stage, acetogenesis. In acetogenesis, volatile fatty acids are consumed by hydrogen, and acetogens produce acetic acid or acetate, hydrogen, and carbon dioxide. The process's efficiency and the hydrogen yield during the acetogenesis of volatile fatty acids are crucial factors in studying anaerobic digestion-based biohydrogen production. The presence of hydrogen-consuming or hydrogenotrophic acetogens is responsible for converting hydrogen and carbon dioxide to acetic acid. However, the production of methane and carbon dioxide, known as methanogenesis, hinders biohydrogen production.
Qiu et al. (2019) studied the role of biochar in enhancing methanogenesis. They reported the positive effect of biochar addition to improve hydrolysis rate, stabilize pH, promote microbial growth, and direct interspecies electron transfer. The latter is an inhibitor of the hydrogen production route to maximize methane synthesis. Anaerobic digestion followed by steam gasification to achieve waste-to-energy was discussed in the study by Zhang et al. (2021c). The researchers focused on a combined anaerobic digestion and steam gasification system to produce methane and hydrogen-rich syngas. Biochar was used to assist in anaerobic digestion to increase the productivity of biomethane, and the produced biomethane was then fed into the gasification process.
Methanogenesis depends on the operating environment, including pH, temperature, carbon-to-nitrogen ratio, retention time, and microbial properties, as explained by Muthudineshkumar and Anand (2019). To maximize biohydrogen production, suppression of methanogenesis is essential by manipulating the properties of the process and environment. One of the recommended ways is the adjustment of pH to reduce methanogenesis.
Yang and Wang (2019) explained that biochar assists biofilm formation, which is essential in microbial growth and removing soluble metabolites inhibitors. Biochar is also a supplier of nutrients such as hydrocarbons that accelerates the expansion of the microbial community. Li et al. (2020a) summarized five significant functions of biochar additives in anaerobic digestion: to enhance the conversion of substrates, buffer pH, reduce oxidation–reduction potential, immobilize cells, and supply minerals. Immobilization of cells regards the adsorption or attachment of microbial community on the biochar. Therefore, high biochar porosity is preferred to provide a larger specific area for microorganism attachment. Recent studies on the performance of biochar-derived additives in anaerobic digestion to produce biohydrogen are summarized in Table 14.
Generally, the addition of biochar in an anaerobic digester is beneficial to improve biohydrogen production, but the efficiency varies extensively. Regarding hydrogen production rate, cornstalk-derived biochar has the most remarkable improvement in Table 14. The hydrogen production rate of the biochar-assisted system was recorded as 22.8 mmol/L h, three times that of the sample without biochar additives, as reported by Zhao et al. (2019a). On the other hand, the maximum increase in hydrogen production yield was 70%, and the yield was 3990 mL/L. Shifting of a maximum point was also noticed when biochar was used and was referred to as lag time reduction in the literature, implying the positive effect of biochar on the rate of anaerobic digestion.
Another significant observation is the higher pH in biochar-assisted systems than without, indicating the function of biochar as a pH regulator. As mentioned, biochar is used as a pH stabilizer in anaerobic digestion. This function is vital to prevent acidification that inhibits microbial community growth and anaerobic digestion. Zhao et al. (2019a) also manipulated hydraulic retention time, and their result shows that with highly effective biochar additives, the availability of substrates is the main limiting factor of hydrogen synthesis.
In the study by Zhao et al. (2021), the quantity and productivity of hydrogen in cornstalk biochar-assisted anaerobic digestion exceed that of the control sample without biochar additives by 93.1% and 75.2%, respectively. They explained that a larger surface area provided by porous biochar is advantageous to allow more attachment of hydrogen-producing microorganisms. Furthermore, the enzymatic activity becomes higher as assisted by biochar, leading to a higher fermentation rate. However, the excessive addition of biochar could lower the enzymatic activities, as indicated by the maximum curve of the experimental result at different concentrations.
Based on the experimental result by Wang et al. (2018), adding biochar can raise hydrogen production yield and rate and shorten the lag time. Among the different biochar used, the highest productivity improvement, recorded as 110%, was achieved with sawdust-derived biochar and sewage sludge biochar produced by pyrolysis at 700 °C and 300 °C, respectively. Nonetheless, sawdust biochar and wheat bran biochar produced by pyrolysis at 700 °C show superiority in hydrogen yield, which is 81 mL/g compared to 65 mg/L of sewage sludge. Conversely, peanut shell biochar impeded hydrogen production, as indicated by the reduced hydrogen yields of all samples despite the increased production of volatile fatty acid. Overall, biochar improves the system's alkalinity and diminishes lag time.
Li et al. (2020a) studied the role of rice straw-derived biochar in anaerobic digestion. They reported an increase of 118.4% and 79.6%, while the yield was recorded as 125.87 mL and 190.1 mL for ethanol and butanoic acid-producing fermentation, respectively. Hydrogen productivity was improved by 220.3% for the ethanol pathway and 159.4% for the butanoic acid pathway. In their study, the result indicates the opposite effects of biochar dosage on hydrogen synthesis of a different pathway. The fabricated biochar additive is said to be conducive to hydrogen production via butanoic acid-producing fermentation. Nevertheless, hydrogen yield via ethanol and butanoic acid pathways is generally lower than acetogenesis.
The authors reported the importance of biochar addition on pH regulation to achieve higher hydrogen yield and production rate. Their result also demonstrated that different biochar dosage leads to different final pH of the system. With higher volatile fatty acid generation, the pH of the biochar-assisted anaerobic digestion system at the final stage is lower because of more accumulated acids. The apparent impact of biochar dosage on the system pH adds to the importance of implementing optimum biochar dosage according to the microbial properties. On the other hand, Wu et al. (2019) reported a relatively lower hydrogen yield improvement when using rice straw biochar, reported as 48.3% only. Energy conversion efficiency also experienced enhancement with the addition of biochar by reducing the heat value of hydrogen generation.
According to Sugiarto et al. (2021), using pinewood-derived biochar increased the hydrogen yield by 107%, while the improvement in the production rate was only 54%. The maximum improvement in yield and production rate with leached biochar using citric acid was 39% and 45%, respectively, lower than the corresponding improvements of 68% and 9%. The study highlighted the role of biochar as a supplier of minerals, particularly potassium, iron, and calcium, which promote the growth of hydrogen-producing bacteria and accelerate the production of acetic acid and butyric acid.
Zhao et al. (2019b) used calcium lignosulfonate, an extract from the sulfite pulp process of wood, for biohydrogen production from sewage sludge. The yield was 262 mL/g glucose higher with biochar addition, recorded as 50.9% than the control. Although raw calcium lignosulfonate impeded hydrogen production due to toxicity and disturbance to electron transfer, the increased porosity of biochar favors hydrogen production. Importantly, biochar releases minerals that help grow microbial communities and activity. Also, the minerals aid in regulating the system's pH to avoid alkalinity depletion.
Referring to the study by Deng et al. (2022), the increase in yield and productivity of hydrogen was not significant for all samples tested, except for the wood biochar-assisted system, with 24.6% and 70.4%, respectively. In the case of draff biochar produced from pyrolysis in carbon dioxide, the hydrogen yield reduction of 25.2% indicates a significant inhibiting effect. Structural complexity could cause slow and difficult anaerobic digestion. Deng et al. (2022) studied using glucose, cellulose, and Laminaria. digitata as substrates. When compared between the hydrogen yield and production rate, hydrogen yield synthesized from glucose with a simpler molecular structure is the highest, followed by Laminaria digitata and cellulose. Structural complexity acts as an inherent inhibiting factor that biochar addition cannot remove.
Yang and Wang (2019) added iron nanoparticles and the fabricated sawdust biochar additives into the anaerobic digester. The researchers reported that the optimal dosage values for metal and biochar were 6 g/L and 4 g/L, respectively, when dry grass was used as the substrate. According to the study, iron nanoparticles were crucial to significantly improving hydrogen production because metallic materials can reduce oxidation–reduction potential. Lower oxidation–reduction potential was found conducive to the growth of hydrogen-producing anaerobic bacteria, leading to a diverse microbial community and a more complete and stable ecosystem. Additionally, iron nanoparticles provide cations necessary for the expansion of the microbial community and the removal of oxygen molecules.
Although the type of substrates contributed to the variation in hydrogen yield and production rate in recent studies, the benefits of biochar additives in biohydrogen production via anaerobic digestion are certain in terms of lag time reduction as well as improvement in maximum yield and production rate. There is potential to integrate biohydrogen production via anaerobic digestion with a pyrolysis-based waste treatment plant to promote sustainability, recycling, and better waste management, as Wang et al. (2018) suggested. The practicality of pyrolysis-anaerobic digestion is still lacking due to the low maturity of biohydrogen production to be profitable and energy efficient when applied on a larger scale. Conventional carbon materials such as graphene have comparable performance with biochar. Nevertheless, the performance of biochar can vary a lot depending on the carbonization process, pre-treatment, and the nature of biomass that cause a difference in porosity and surface functional groups.
On the other hand, acidification with the accumulation of volatile fatty acids is impeded by biochar, resulting in the stabilization of pH and promotion of microbial growth. As most studies depict, acetic acid is the dominant species of volatile fatty acids produced in anaerobic digestion. However, the share of butyrate increases simultaneously with higher biochar dosage. In some cases, butyrate is the most produced volatile fatty acid in anaerobic digestion. This is because the types of additives, microbial properties, and substrates influence the rate of different fermentation pathways.
To sum up, biohydrogen yield and production rate in anaerobic digestion of organic matter depend on the characteristics of biochar additives and the substrate and microbial properties. Variable performance that relies on multiple factors adds to the difficulty in implementing and scaling biohydrogen production in the waste treatment process to achieve sustainable and low-cost waste to energy.
Biomass energy and the environment
Biomass energy is known as transition energy and is a promising substitute for fossil energy in future. However, biomass energy falls short when considering various issues associated with feedstock selection, energy production methods, and potential environmental concerns. Therefore, the advantages and disadvantages of biomass energy from different aspects are discussed in this section.
Biomass energy is advantageous due to feedstock's variety, abundance, and renewability. Generally, vegetation-derived biomass energy is widely known for being carbon neutral, as plants consume carbon dioxide for living and growth. Nonetheless, this claim is only true if the emission rate is equivalent to the carbon or carbon sink capture rate. Woody vegetation is the primary biomass source in the current applications of biomass energy generation. In most applications, wood is converted to biochar for heat and electricity production. Therefore, forest management is often the focal point in the discussion of the carbon neutrality of biomass energy. A carbon cycle of forest biomass energy is illustrated in Fig. 18 by referring to Birdsey et al. (2018) and Ahmadi et al. (2020).
Carbon biomass cycle. The carbon cycle includes the land sector, forest sector, and the consumption of fossil fuels. Achieving carbon neutrality for biomass energy requires that carbon capture occurs at the same rate as carbon release during biomass energy production. Woody biomass is a significant source of energy production, so forest management is crucial for achieving carbon neutrality in biomass energy. Effective forest management requires continuous efforts from government, private agencies, and the community. Nevertheless, the impacts of deforestation are complex and can have permanent effects on ecosystems and the environment, often requiring a lengthy period of replantation
Pisciotta et al. (2022) suggested that biomass energy production has lower carbon dioxide emission intensity because the common biomass sources are the plants and woods that consume carbon dioxide when growing. This allows reducing carbon dioxide emissions to achieve a net zero carbon footprint in bioenergy production. Carbon neutrality of forest-based biomass energy is achieved with sufficiently large forests to provide carbon sinks that capture biogenic carbon. This greatly relies on the balance between harvest and replantation. Effective forest management requires continuous efforts in terms of the establishment and enforcement of policies as well as investment. The significantly longer time taken to restore a forest compared to logging and harvesting can be attributed to inadequate efforts and the lack of recognition of the role of forests in carbon sequestration.
Lin and Ge (2020) stated three functions of forests: carbon sinks to reduce atmospheric carbon content, biomass energy production to replace fossil energy, and wood products to generate economic benefits. They suggested that a green economy is realized by applying charges on carbon emissions which helps bring the balance in the biogenic carbon cycle. Besides the division of forests for different areas for various industries, including wood products and bioenergy, other areas, such as carbon sink forest areas and ecological forestry protection areas, shall be determined and protected to retain both the economic and ecological function of forests. They added that evaluating the size of available forest carbon sinks is essential to ensure the practicality of the carbon emission reduction targets.
While carbon neutrality can be achieved with forest management, impacts on the regional ecosystem and climate brought by deforestation are undeniable when considering the time taken to completely restore the forest. Deforestation is known for its adverse impacts on global and local climate as well as water cycles. Besides, soil health and integrity are affected due to the changes in properties and functionality. Veldkamp et al. (2020) indicated that soil biology is involved in addition to changes in dynamic soil properties such as pH, base saturation, bulk density, carbon-to-nitrogen ratio, soil organic carbon, and effective cation exchange capacity. The adverse impacts on the soil ecosystem can alter soil biology.
On the other hand, permanent changes in the soil properties are resulted during the period of reforestation. Again, the restoration process can be limited by the lack of legal actions and investment from the government and voluntary action from non-governmental organizations and the community. Deforestation and land use change for preparing energy crop plantation are not followed by reforestation. This leads to the loss of natural habitat besides severe disturbance to the local ecosystem and natural cycles. In the biomass energy industry, issues to be addressed are food insecurity caused by the promotion of food crop use for biofuel production. In addition, the plantation of energy crops also reduces food cropland availability and disturbs soil organic carbon balance affecting soil health, as stated by Liu et al. (2018). They stated the lack of addressing the abovementioned issues caused by land use change and occupation for energy crop plantations in China.
The carbon neutrality of biomass energy has been doubted due to the energy intensity of the biofuel preparation process. Thermal treatment at high temperatures is a common and effective method of preparing gaseous, liquid, and solid biofuels, as discussed in the previous sections. In larger-scale biofuel production, energy demand and carbon emission are significant, which adds to the importance of fuel choice in biofuel production. The high energy demand for biofuel production leads to releasing geological carbon from burning fossil fuels.
A mixture of liquid biofuel and fossil fuel is widely applied in transportation due to the similarity between the two energy sources that allow direct blending. Ližbetin et al. (2018) conducted a comparison study between fatty acid methyl ester and fossil diesel. The former requires greater energy input for fuel production, owing to the high energy intensity of the production process. Conversely, fatty acid methyl ester releases only 31% of the greenhouse gases emitted from burning fossil diesel.
The carbon footprint of biomass energy production also depends on the type of transport used and the traveling distance. Beagle and Belmont (2019) reported the effect of the transportation of biomass feedstocks in terms of distance and emission factor of transportation used on the overall carbon footprint of a biomass-based or co-firing power plant. Similarly, Ilari et al. (2022) reported that in the entirety of biomass energy generation, including feedstocks collection, transportation of materials, processing and storing materials in the plant, electricity generation, and dumping of residual ash, a major part of carbon dioxide is emitted from the transportation and power generation process with a share of 34% and 20%, respectively. Fan et al. (2018) stated that despite biomass energy production being carbon neutral, the carbon footprint of acquiring biomass, including harvesting, collecting, transporting, and storing, shall be considered. In this matter, Pergola et al. (2020) believed that the supply should be obtained from nearby sources such as local forest harvesting and sawmilling. Besides, they mentioned the importance of evaluating the correlation between biomass removal and soil quality and the local ecosystem.
A waste-to-energy system such as incineration, gasification, and anaerobic digestion produces biomass energy without consuming natural resources. It is considered a relatively environmentally benign waste management method that realizes waste reduction and is a cheap biomass energy source. Wastewater and solid waste treatments have become profitable with renewable electricity and heat production. Waste-to-energy system is promoted to replace landfilling, which carries high environmental pollution risks and is difficult to monitor or control. However, efforts are also taken to reduce the environmental impacts of landfilling.
The waste-to-energy system has been implemented, and new technology to increase energy efficiency and productivity is being explored. Implementing a waste-to-energy system is essential to reduce the reliance of biomass energy production on forest biomass and food crops. The utilization of wastes as the feedstock of biomass energy production does not cause global issues related to food security, land, and water, as well as timber, food, and agricultural industries. Table 15 shows the production of municipal wastes and the global population, while Fig. 19 shows different waste management techniques in the selected countries.
Waste management profiles in some countries in 2018, according to Organization for Economic Cooperation and Development (2022). Data show the variety of techniques used in different countries. The global population is expected to increase from 7.942 billion to 9.687 billion between 2022 and 2050. This leads to potential growth in waste production. In this scenario, a waste-to-energy system is a promising method of simultaneous global waste management and renewable energy production
Organization for Economic Cooperation and Development (2022) stated that in OECD (Organization for Economic Cooperation and Development) countries, global municipal waste rose from 682,495.3 kilotons to 732,105.5 kilotons between 2015 and 2020. Note that municipal waste production in non-OECD countries, including China, Brazil, Cyprus, Indonesia, and Russia, is excluded from the table due to incomplete data. The world population also increased from 7.35 billion to 7.76 billion in the same period. The global population between United Nations (2022) forecasted the global population increase from 7.942 billion to 9.687 billion between 2022 and 2050. As the production of municipal waste increases simultaneously with the global population, the waste-to-energy system is the ultimate alternative to tackle global waste management and renewable energy production.
Preparing suitable feedstock for thermal-based waste-to-energy systems, such as incineration, gasification, and pyrolysis, can be challenging, costly, and energy-intensive due to the variability in quantity, composition, and physicochemical properties of waste. These properties can be affected by various factors such as weather, season, and waste management practices and can significantly impact the quality of waste in terms of moisture content and calorific value.
One crucial environmental impact of biomass energy is the emission of nitrogen oxides, sulfur oxides, and carbon monoxide in burning biomass sourced from forests and wastes. Referring to Chand Malav et al. (2020), thermal treatment, especially incineration, is a source of ash, sulfur dioxide, nitrogen dioxide, furans, heavy metals, carcinogens, and particulates, causing health threats to the public. Pyrolysis and gasification of wastes also release volatiles, heavy metals, flue gas, sulfur, and halogens-containing compounds into the atmosphere. Biological waste-to-energy methods such as aerobic decomposition, anaerobic digestion, and fermentation of organic wastes face several challenges, including high cost, prolonged reaction time, complex operating conditions, difficulty in scaling up, large space requirements, risk of disease spreading, and high power requirements, as reported by Ma and Liu (2019) and Chand Malav et al. (2020).
Overall, vegetation, municipal wastes, and food wastes are highly potential to achieve sustainable and reduced carbon emission biomass energy production due to their abundance and renewability. However, municipal and food wastes are less of a quality feedstock than woody biomass, leading to low energy productivity and cost inefficiency. While woody biomass is an excellent biomass energy feedstock, intensive investment, and efforts are necessary to eliminate excessive carbon emissions from imbalanced harvest replantation. Importantly, all identified biomass energy sources possess significant environmental pollution risks with the production of other pollutants besides carbon dioxide.
Biomass energy certainly is helpful in global carbon emission cutting. Reducing geological carbon emissions by substituting fossil energy for biomass energy should be carefully studied, executed, and expanded.
Conclusion
The article comprehensively overviews biomass conversion pathways, including thermochemical and biological conversion processes. The performance and share of biomass energy are also discussed, along with the application of biochar in renewable energy production. The article also highlights the environmental impacts of biomass energy, particularly emissions of nitrogen oxides, sulfur oxides, and carbon monoxide.
Biochemical and thermochemical conversion methods are the two main approaches for converting biomass into biofuels. Biochemical conversion involves using microorganisms or enzymes to break down biomass into simpler sugars and fermenting them to produce ethanol or other biofuels. Thermochemical conversion, however, involves heating biomass without oxygen to produce a mixture of gases, liquids, and solids. The gases can produce synthetic natural gas or hydrogen, while the liquids can be used as bio-oil or upgraded to produce transportation fuels. Both methods have advantages and disadvantages, and the choice of method depends on various factors such as the type of biomass, the desired end product, and the economic feasibility of the process. However, biofuels produced from biomass have the potential to reduce greenhouse gas emissions and dependence on fossil fuels. With the growing demand for sustainable and environmentally friendly transportation fuels, the biofuels market is projected to grow significantly in the coming years. The review also examined the potential of biochar in renewable energy, highlighting its capacity to serve as a catalyst in various processes such as biodiesel and biohydrogen production, as well as its involvement in water splitting, methane to hydrogen conversion, electricity generation in microbial fuel cells, and hydrogen production from anaerobic digestion.
References
Abdulyekeen KA, Umar AA, Patah MFA, Daud WM (2021) Torrefaction of biomass: production of enhanced solid biofuel from municipal solid waste and other types of biomass. Renew Sustain Energy Rev 150:111436
Abraham A, Mathew AK, Park H, Choi O, Sindhu R, Parameswaran B, Pandey A, Park JH, Sang BI (2020) Pretreatment strategies for enhanced biogas production from lignocellulosic biomass. Bioresour Technol 301:122725
Abubakar AM (2022) Biodigester and feedstock type: characteristic, selection, and global biogas production. J Eng Res Sci 1(2):170–187
Agu CE, Tokheim L-A, Pfeifer C, Moldestad BM (2019) Behaviour of biomass particles in a bubbling fluidized bed: a comparison between wood pellets and wood chips. Chem Eng J 363:84–98
Ahmadi L, Kannangara M, Bensebaa F (2020) Cost-effectiveness of small scale biomass supply chain and bioenergy production systems in carbon credit markets: a life cycle perspective. Sustain Energy Technol Assess 37:100627
Ajanovic A, Sayer M, Haas R (2022) The economics and the environmental benignity of different colors of hydrogen. Int J Hydrog Energy 47:24136–24154
Akbari M, Oyedun AO, Gemechu E, Kumar A (2021) Comparative life cycle energy and greenhouse gas footprints of dry and wet torrefaction processes of various biomass feedstocks. J Environ Chem Eng 9(4):105415
Akhbari A, Ibrahim S (2022) Bioenergy recovery from food waste through dark fermentation direction
Aliyu A, Lee J, Harvey AP (2021) Microalgae for biofuels: a review of thermochemical conversion processes and associated opportunities and challenges. Bioresour Technol Rep 15:100694
Alkharabsheh HM, Seleiman MF, Battaglia ML, Shami A, Jalal RS, Alhammad BA, Almutairi KF, Al-Saif AM (2021) Biochar and its broad impacts in soil quality and fertility, nutrient leaching and crop productivity: a review. Agronomy 11(5):993
Almatouq A, Babatunde AO, Khajah M, Webster G, Alfodari M (2020) Microbial community structure of anode electrodes in microbial fuel cells and microbial electrolysis cells. J Water Process Eng 34:101140
AlNouss A, Parthasarathy P, Shahbaz M, Al-Ansari T, Mackey H, McKay G (2020) Techno-economic and sensitivity analysis of coconut coir pith-biomass gasification using ASPEN PLUS. Appl Energy 261:114350
Alvarez A, Migoya S, Menendez R, Gutierrez G, Pizarro C, Bueno JL (2021) Torrefaction of Short Rotation Coppice Willow. characterization, hydrophobicity assessment and kinetics of the process. Fuel 295:120601
Alvarez-Guzmán CL, Cisneros-de la Cueva S, Balderas-Hernández VE, Smoliński A, De León-Rodríguez A (2020) Biohydrogen production from cheese whey powder by Enterobacter asburiae: effect of operating conditions on hydrogen yield and chemometric study of the fermentative metabolites. Energy Rep 6:1170–1180
Amenaghawon AN, Anyalewechi CL, Okieimen CO, Kusuma HS (2021) Biomass pyrolysis technologies for value-added products: a state-of-the-art review. Environ Dev Sustain, pp 1–55
Anand A, Pathak S, Kumar V, Kaushal P (2022) Biochar production from crop residues, its characterization and utilization for electricity generation in India. J Clean Prod 368:133074
Anto S, Karpagam R, Renukadevi P, Jawaharraj K, Varalakshmi P (2019) Biomass enhancement and bioconversion of brown marine microalgal lipid using heterogeneous catalysts mediated transesterification from biowaste derived biochar and bionanoparticle. Fuel. https://doi.org/10.1016/j.fuel.2019.115789
Ayub HMU, Ahmed A, Lam SS, Lee J, Show PL, Park Y-K (2022) Sustainable valorization of algae biomass via thermochemical processing route: an overview. Bioresour Technol 344:126399
Babinszki B, Sebestyén Z, Jakab E, Kőhalmi L, Bozi J, Várhegyi G, Wang L, Skreiberg Ø, Czégény ZJBT (2021) Effect of slow pyrolysis conditions on biocarbon yield and properties: characterization of the volatiles. Biores Technol 338:125567
Baeyens J, Zhang H, Nie J, Appels L, Dewil R, Ansart R, Deng YJR, Reviews SE (2020) Reviewing the potential of bio-hydrogen production by fermentation. Renew Sustain Energy Rev 131:110023
Barontini F, Frigo S, Gabbrielli R, Sica P (2021) Co-gasification of woody biomass with organic and waste matrices in a down-draft gasifier: an experimental and modeling approach. Energy Convers Manage 245:114566
Barskov S, Zappi M, Buchireddy P, Dufreche S, Guillory J, Gang D, Hernandez R, Bajpai R, Baudier J, Cooper RJRE (2019) Torrefaction of biomass: a review of production methods for biocoal from cultured and waste lignocellulosic feedstocks. Renew Energy 142:624–642
Basumatary S, Nath B, Das B, Kalita P, Basumatary B (2021) Utilization of renewable and sustainable basic heterogeneous catalyst from Heteropanax fragrans (Kesseru) for effective synthesis of biodiesel from Jatropha curcas oil. Fuel 286:119357
Beagle E, Belmont E (2019) Comparative life cycle assessment of biomass utilization for electricity generation in the European Union and the United States. Energy Policy 128:267–275
Behera B, Dey B, Balasubramanian P (2020) Algal biodiesel production with engineered biochar as a heterogeneous solid acid catalyst. Bioresour Technol 310:123392
Beims RF, Hu Y, Shui H, Xu CC (2020) Hydrothermal liquefaction of biomass to fuels and value-added chemicals: products applications and challenges to develop large-scale operations. Biomass Bioenerg 135:105510
Ben Atitallah I, Antonopoulou G, Ntaikou I, Soto Beobide A, Dracopoulos V, Mechichi T, Lyberatos GJM (2022) A comparative study of various pretreatment approaches for bio-ethanol production from Willow Sawdust, using co-cultures and mono-cultures of different yeast strains. Molecules 27(4):1344
Bensalah F, Pézard J, Haddour N, Erouel M, Buret F, Khirouni K (2021) Carbon nano-fiber/PDMS composite used as corrosion-resistant coating for copper anodes in microbial fuel cells. Nanomaterials 11(11):3144
Bertelsen N, Vad Mathiesen B (2020) EU-28 residential heat supply and consumption: historical development and status. Energies 13(8):1894
Bhakta AK, Fiorenza R, Jlassi K, Mekhalif Z, Abdullah Ali AM, Chehimi MM (2022) The emerging role of biochar in the carbon materials family for hydrogen production. Chem Eng Res Des 188:209–228
Bhatia SK, Jagtap SS, Bedekar AA, Bhatia RK, Rajendran K, Pugazhendhi A, Rao CV, Atabani A, Kumar G, Yang Y-H (2021) Renewable biohydrogen production from lignocellulosic biomass using fermentation and integration of systems with other energy generation technologies. Sci Total Environ 765:144429
Bhoi P, Ouedraogo A, Soloiu V, Quirino R (2020) Recent advances on catalysts for improving hydrocarbon compounds in bio-oil of biomass catalytic pyrolysis. Renew Sustain Energy Rev 121:109676
Birdsey R, Duffy P, Smyth C, Kurz WA, Dugan AJ, Houghton R (2018) Climate, economic, and environmental impacts of producting wood for bioenergy. Environ Res Lett 3:050201
Bogoviz AV, Lobova SV, Alekseev AN (2020) Current state and future prospects of hydro energy in Russia. Int J Energy Econ Policy. https://doi.org/10.32479/ijeep.8968
Bošnjaković M, Sinaga N (2020) The perspective of large-scale production of algae biodiesel. Appl Sci 10:8181
BP-Statistics (2022) BP statistical review of world energy 71st Edition. https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2022-full-report.pdf. London, United Kingdom
Brachi P, Chirone R, Miccio M, Ruoppolo GJPT (2019) Fluidized bed torrefaction of biomass pellets: a comparison between oxidative and inert atmosphere. Powder Technol 357:97–107
Brindhadevi K, Shanmuganathan R, Pugazhendhi A, Gunasekar P, Manigandan S (2021) Biohydrogen production using horizontal and vertical continuous stirred tank reactor-a numerical optimization. Int J Hydrog Energy 46(20):11305–11312
Briones-Hidrovo A, Copa J, Tarelho LA, Gonçalves C, da Costa TP, Dias AC (2021) Environmental and energy performance of residual forest biomass for electricity generation: gasification vs. combustion. J Clean Prod 289:125680
Byun J, Cha Y-L, Park S-M, Kim K-S, Lee J-E, Kang Y-GJE (2020) Lignocellulose pretreatment combining continuous alkaline single-screw extrusion and ultrasonication to enhance biosugar production. Energies 13(21):5636
Cai Y, Zheng Z, Schäfer F, Stinner W, Yuan X, Wang H, Cui Z, Wang X (2021) A review about pretreatment of lignocellulosic biomass in anaerobic digestion: achievement and challenge in Germany and China. J Clean Prod 299:126885
Calì G, Deiana P, Bassano C, Meloni S, Maggio E, Mascia M, Pettinau AJE (2020) Syngas production, clean-up and wastewater management in a demo-scale fixed-bed updraft biomass gasification unit. Energies 13(10):2594
Cao Y, Fu L, Mofrad A (2019) Combined-gasification of biomass and municipal solid waste in a fluidized bed gasifier. J Energy Inst 92(6):1683–1688
Cao L, Iris K, Xiong X, Tsang DC, Zhang S, Clark JH, Hu C, Ng YH, Shang J, Ok YS (2020) Biorenewable hydrogen production through biomass gasification: a review and future prospects. Environ Res 186:109547
Chaijak P, Sato C, Lertworapreecha M, Sukkasem C, Boonsawang P, Paucar N (2020) Potential of biochar-anode in a ceramic-separator microbial fuel Cell (CMFC) with a laccase-based air cathode. Pol J Environ Stud 29:499–503
Chakraborty I, Sathe SM, Dubey BK, Ghangrekar MM (2020) Waste-derived biochar: applications and future perspective in microbial fuel cells. Biosour Technol 312:123587
Chand Malav L, Yadav KK, Gupta N, Kumar S, Sharma GK, Krishnan S, Rezania S, Kamyab H, Pham QB, Yadav S, Bhattacharyya S, Yadav VK, Bach Q-V (2020) A review on municipal solid waste as a renewable source for waste-to-energy project in India: current practices, challenges, and future opportunities. J Clean Prod 277:123227
Changmai B, Vanlalveni C, Ingle AP, Bhagat R, Rokhum SL (2020) Widely used catalysts in biodiesel production: a review. RSC Adv 10(68):41625–41679
Chaturvedi V, Goswami RK, Verma P (2020) Genetic engineering for enhancement of biofuel production in microalgae. In: Verma P (ed) Biorefineries: a step towards renewable and clean energy. Springer Singapore, Singapore, pp 539–559
Chen D, Wang XN, Zhang XQ, Yang Y, Xu Y, Qian GR (2019a) Facile fabrication of mesoporous biochar/ZnFe2O4 composite with enhanced visible-light photocatalytic hydrogen evolution. Int J Hydrog Energy 44:19967–19977
Chen JY, Xie P, Zhang ZP (2019b) Reduced graphene oxide/polyacrylamide composite hydrogel scaffold as biocompatible anode for microbial fuel cell. Chem Eng J 361:615–624
Chen L, Nakamoto R, Kudo S, Asano S, Hayashi JI (2019c) Biochar-assisted water electrolysis. Energy Fuels 33(11):11246–11252
Chen S, Patil SA, Keith Brown R, Schröder U (2019d) Strategies for optimizing the power output of microbial fuel cells: transitioning from fundamental studies to practical implementation. Appl Energy 233–234:15–28
Chen D, Chen F, Cen K, Cao X, Zhang J, Zhou JJF (2020) Upgrading rice husk via oxidative torrefaction: characterization of solid, liquid, gaseous products and a comparison with non-oxidative torrefaction. Fuel 275:117936
Chen W-H, Eng CF, Lin Y-Y, Bach Q-V, Ashokkumar V, Show P-L (2021a) Two-step thermodegradation kinetics of cellulose, hemicelluloses, and lignin under isothermal torrefaction analyzed by particle swarm optimization. Energy Convers Manag 238:114116
Chen W-H, Lin B-J, Lin Y-Y, Chu Y-S, Ubando AT, Show PL, Ong HC, Chang J-S, Ho S-H, Culaba AB (2021b) Progress in biomass torrefaction: principles, applications and challenges. Prog Energy Combust Sci 82:100887
Chen H, Wu J, Huang R, Zhang W, He W, Deng Z, Han Y, Xiao B, Luo H, Qu WJC (2022a) Effects of temperature and total solid content on biohydrogen production from dark fermentation of rice straw: Performance and microbial community characteristics. Chemosphere 286:131655
Chen ZJ, Zheng RJ, Wei WF, Wei W, Zou W, Li J, Ni BJ, Chen H (2022c) Recycling spent water treatment adsorbents for efficient electrocatalytic water oxidation reaction. Resour Conserv Recycl 178:106037
Cheng F, Li XW (2018) Preparation and application of biochar-based catalysts for biofuel production. Catalysts 8:346
Choi HI, Lee JS, Choi JW, Shin YS, Sung YJ, Hong ME, Kwak HS, Kim CY, Sim SJ (2019) Performance and potential appraisal of various microalgae as direct combustion fuel. Bioresour Technol 273:341–349
Dahunsi SO (2019) Mechanical pretreatment of lignocelluloses for enhanced biogas production: methane yield prediction from biomass structural components. Bioresour Technol 280:18–26
Daimary N, Eldiehy KSH, Boruah P, Deka D, Bora U, Kakati BK (2022) Potato peels as a sustainable source for biochar, bio-oil and a green heterogeneous catalyst for biodiesel production. J Environ Chem Eng 10:107108
Deng LF, Zhang YY, Wang YZ, Yuan HR, Chen Y, Wu YF (2021) In situ N-, P- and Ca-codoped biochar derived from animal bones to boost the electrocatalytic hydrogen evolution reaction. Resour Conserv Recycling. https://doi.org/10.1016/j.resconrec.2021.105568
Deng C, Lin RC, Kang XH, Wu BT, Wall D, Murphy JD (2022) Improvement in biohydrogen and volatile fatty acid production from seaweed through addition of conductive carbon materials depends on the properties of the conductive materials. Energy 239:122188
Din MI, Iqbal M, Hussain Z, Khalid R (2020) Bioelectricity generation from waste potatoes using single chambered microbial fuel cell. Energy Sour Part A Recovery Utilization Environ Eff. https://doi.org/10.1080/15567036.2020.1797944
Du Y, Ying Z, Zheng X, Dou B, Cui G (2022) Correlating electrochemical biochar oxidation with electrolytes during biochar-assisted water electrolysis for hydrogen production. Fuel. https://doi.org/10.1016/j.fuel.2022.126957
Durak H, Genel SJ (2020) Characterization of bio-oil and bio-char obtained from black cumin seed by hydrothermal liquefaction: investigation of potential as an energy source. Energy Sour Part A Recovery Utilization Environ Eff. https://doi.org/10.1016/j.enpol.2020.111906
Ebadian M, van Dyk S, McMillan JD, Saddler J (2020) Biofuels policies that have encouraged their production and use: an international perspective. Energy Policy 147:111906
Emebu S, Pecha J, Janáčová DJR, Reviews SE (2022) Review on anaerobic digestion models: model classification & elaboration of process phenomena. Renew Sustain Energy Rev 160:112288
Eswari AP, Kannah RY, Kavitha S, Banu JR (2023) Recent insight into anaerobic digestion of lignocellulosic biomass for cost effective bioenergy generation. e Prime Adv Electr Eng Electron Energy. https://doi.org/10.1016/j.prime.2023.100119
Fahmy TY, Fahmy Y, Mobarak F, El-Sakhawy M, Abou-Zeid RE (2020) Biomass pyrolysis: past, present, and future. Environ Dev Sustain 22:17–32
Fan JM, Hong H, Jin HG (2018) Biomass and coal co-feed power and SNG polygeneration with chemical looping combustion to reduce carbon footprint for sustainable energy development: process simulation and thermodynamic assessment. Renew Energy 125:260–269
Fan JL, Wang JX, Hu JW, Yang Y, Wang Y (2021) Will China achieve its renewable portfolio standard targets? An analysis from the perspective of supply and demand. Renew Sustain Energy Rev 138:110510
Fang P, Gong Z, Wang Z, Wang Z, Meng F (2019) Study on combustion and emission characteristics of microalgae and its extraction residue with TG-MS. Renew Energy 140:884–894
Farghali M, Ap Y, Mohamed IMA, Iwasaki M, Tangtaweewipat S, Ihara I, Sakai R, Umetsu K (2021) Thermophilic anaerobic digestion of Sargassum fulvellum macroalgae: biomass valorization and biogas optimization under different pre-treatment conditions. J Environ Chem Eng 9(6):106405
Farghali M, Osman AI, Umetsu K, Rooney DW (2022) Integration of biogas systems into a carbon zero and hydrogen economy: a review. Environ Chem Lett 20(5):2853–2927
Farghali M, Mohamed IMA, Osman AI, Rooney DW (2023a) Seaweed for climate mitigation, wastewater treatment, bioenergy, bioplastic, biochar, food, pharmaceuticals, and cosmetics: a review. Environ Chem Lett 21(1):97–152
Farghali M, Osman AI, Mohamed IMA, Chen Z, Chen L, Ihara I, Yap P-S, Rooney DW (2023c) Strategies to save energy in the context of the energy crisis: a review. Environ Chem Lett 23:1–37
Farghali M, Osman AI, Chen Z, Abdelhaleem A, Ihara I, Mohamed IMA, Yap P-S, Rooney DW (2023b) Social, environmental, and economic consequences of integrating renewable energies in the electricity sector: a review. Environ Chem Lett
Feng Y, Qiu K, Zhang Z, Li C, Rahman MM, Cai JJE (2022) Distributed activation energy model for lignocellulosic biomass torrefaction kinetics with combined heating program. Energy 239:122228
Ferreira TB, Rego GC, Ramos LR, de Menezes CA, Silva EL (2020) Improved dark fermentation of cane molasses in mesophilic and thermophilic anaerobic fluidized bed reactors by selecting operational conditions. J Energy Res 44(13):10442–10452
Gemechu ED, Kumar A (2021) The environmental performance of hydrogen production pathways based on renewable sources. In: Ren JZ (ed) Renewable-energy-driven future. Academic Press, Cambridge, pp 375–406
Gielen D, Boshell F, Saygin D, Bazilian MD, Wagner N, Gorini R (2019) The role of renewable energy in the global energy transformation. Energ Strat Rev 24:38–50
González-Arias J, Gómez X, Gonzalez-Castano M, Sanchez ME, Rosas J, Cara-Jiménez JJE (2022) Insights into the product quality and energy requirements for solid biofuel production: a comparison of hydrothermal carbonization, pyrolysis and torrefaction of olive tree pruning. Energy 238:122022
Gorgec FK, Karapinar I (2019) Biohydrogen production from hydrolyzed waste wheat by dark fermentation in a continuously operated packed bed reactor: the effect of hydraulic retention time. Int J Hydrog Energy 44(1):136–143
Gutierrez-Gomez AC, Gallego AG, Palacios-Bereche R, de Campos Leite JT, Neto AMP (2021) Energy recovery potential from Brazilian municipal solid waste via combustion process based on its thermochemical characterization. J Clean Prod 293:126145
Hameed Z, Aslam M, Khan Z, Maqsood K, Atabani A, Ghauri M, Khurram MS, Rehan M, Nizami A-S (2021) Gasification of municipal solid waste blends with biomass for energy production and resources recovery: current status, hybrid technologies and innovative prospects. Renew Sustain Energy Rev 136:110375
Hanchate N, Ramani S, Mathpati C, Dalvi VH (2021) Biomass gasification using dual fluidized bed gasification systems: a review. J Clean Prod 280:123148
Harun K, Adhikari S, Jahromi H (2020) Hydrogen production via thermocatalytic decomposition of methane using carbon-based catalysts. RSC Adv 10(67):40882–40893
Hashemi SS, Karimi K, Mirmohamadsadeghi SJE (2019) Hydrothermal pretreatment of safflower straw to enhance biogas production. Energy 172:545–554
He Z, Sun Y, Cheng S, Jia Z, Tu R, Wu Y, Shen X, Zhang F, Jiang E, Xu XJF (2021) The enhanced rich H2 from co-gasification of torrefied biomass and low rank coal: The comparison of dry/wet torrefaction, synergetic effect and prediction. Fuel 287:119473
He Y, Zhang S, Liu D, Xie X, Li BJE (2023) Effect of biomass particle size on the torrefaction characteristics in a fixed-bed reactor. Energies 16(3):1104
Hu Y, Gu Z, Li W, Xu CC (2020) Alkali-catalyzed liquefaction of pinewood sawdust in ethanol/water co-solvents. Biomass Bioenerg 134:105485
Hu Y, Pang K, Cai L, Liu ZJE (2021) A multi-stage co-gasification system of biomass and municipal solid waste (MSW) for high quality syngas production. Energy 221:119639
Humagain G, MacDougal K, MacInnis J, Lowe JM, Coridan RH, MacQuarrie S, Dasog M (2018) Highly efficient, biochar-derived molybdenum carbide hydrogen evolution electrocatalyst. Adv Energy Mater. https://doi.org/10.1002/aenm.201801461
Hung YH, Liu TY, Chen HY (2019) Renewable coffee waste-derived porous carbons as anode materials for high-performance sustainable microbial fuel cells. ACS Sustain Chem Eng 7:16991–16999
Ighalo JO, Iwuchukwu FU, Eyankware OE, Iwuozor KO, Olotu K, Bright OC, Igwegbe CAJCT, Policy E (2022) Flash pyrolysis of biomass: a review of recent advances. Clean Technol Environ Policy 24(8):2349–2363
Ilari A, Duca D, Boakye-Yiadom KA, Gasperini T, Toscano G (2022) Carbon footprint and feedstock quality of a real biomass power plant fed with forestry and agricultural residues. Resources 11(2):7
International Energy Agency (2021) Global energy review: CO2 emissions in 2021. OECD Publishing, Paris
International Renewable Energy Agency (2022) World energy transitions outlook 2022: 1.5C pathway. International Renewable Energy Agency, Abu Dhabi
Isemin R, Marias F, Muratova N, Kuzmin S, Klimov D, Mikhalev A, Milovanov O, Brulé M, Tabet FJP (2021) Wet torrefaction of poultry litter in a pilot unit: a numerical assessment of the process parameters. Processes 9(10):1835
Janajreh I, Elkadi K, Makanjuola O, Elagroudy S (2019) Bio-hydrogen production via reforming of anaerobic digestion biogas
Jatoi AS, Shah AA, Ahmed J, Rehman S, Sultan SH, Shah AK, Raza A, Mubarak NM, Hashmi Z, Usto MA (2022) Hydrothermal liquefaction of lignocellulosic and protein-containing biomass: a comprehensive review. Catalysts 12(12):1621
Jeong Y-S, Choi Y-K, Park K-B, Kim J-SJE (2019) Air co-gasification of coal and dried sewage sludge in a two-stage gasifier: Effect of blending ratio on the producer gas composition and tar removal. Energy 185:708–716
Jiang P, Fan YV, Klemeš JJ (2021a) Impacts of COVID-19 on energy demand and consumption: challenges, lessons and emerging opportunities. Appl Energy 285:116441
Jiang TT, Ji PJ, Shi Y, Ye Z, Jin Q (2021b) Efficiency assessment of green technology innovation of renewableenergy enterprises in China: a dynamic data envelopment analysisconsidering undesirable output. Clean Technol Environ Policy 23(3):1–11
Kabutey FT, Zhao QL, Wei LL, Ding J, Antwi P, Quashie FK, Wang W (2019) An overview of plant microbial fuel cells (PMFCs): configurations and applications. Renew Sustain Energy Rev 110:402–414
Kainthola J, Kalamdhad AS, Goud VVJPB (2019) A review on enhanced biogas production from anaerobic digestion of lignocellulosic biomass by different enhancement techniques. Process Biochem 84:81–90
Kandasamy S, Zhang B, He Z, Chen H, Feng H, Wang Q, Wang B, Ashokkumar V, Siva S, Bhuvanendran NJE (2020) Effect of low-temperature catalytic hydrothermal liquefaction of Spirulina platensis. Energy 190:116236
Kanwal F, Ahmed A, Jamil F, Rafiq S, Ayub HU, Ghauri M, Khurram MS, Munir S, Inayat A, Abu Bakar MS (2021) Co-combustion of blends of coal and underutilised biomass residues for environmental friendly electrical energy production. Sustainability 13(9):4881
Karrabi M, Ranjbar FM, Shahnavaz B, Seyedi SJF (2023) A comprehensive review on biogas production from lignocellulosic wastes through anaerobic digestion: an insight into performance improvement strategies. Fuel 340:127239
Kaza S, Yao L, Bhada-Tata P, Van Woerden F (2018) What a waste. World Bank Publications, Washington, D.C.
Khalid Z, Siddique MNI, Nayeem A, Adyel TM, Ismail S, Ibrahim MZ (2021) Biochar application as sustainable precursors for enhanced anaerobic digestion: a systematic review. J Environ Chem Eng 9(4):105489
Khan HM, Iqbal T, Ali CH, Javaid A, Cheema II (2020) Sustainable biodiesel production from waste cooking oil utilizing waste ostrich (Struthio camelus) bones derived heterogeneous catalyst. Fuel 277:118091
Kim MY, Lee DJ, Jung SY, Chang SX, Lin KYA, Bhatnagar A, Kwon EE, Tsang YF (2021) Valorization of peanut wastes into a catalyst in production of biodiesel. Int J Energy Res. https://doi.org/10.1002/er.7248
Kiminaitė I, Lisauskas A, Striūgas N, Kryževičius Ž (2022) Fabrication and characterization of environmentally friendly biochar anode. Energies 15(1):112
Kotarska K, Dziemianowicz W, Świerczyńska A (2021) The effect of detoxification of lignocellulosic biomass for enhanced methane production. Energies 14(18):5650
Kour D, Rana KL, Yadav N, Yadav AN, Rastegari AA, Singh C, Negi P, Singh K, Saxena AK (2019) Technologies for biofuel production: current development, challenges, and future prospects. In: Rastegari AA, Yadav AN, Gupta A (eds) Prospects of renewable bioprocessing in future energy systems. Springer International Publishing, Cham, pp 1–50
Kucharska K, Rybarczyk P, Hołowacz I, Konopacka-Łyskawa D, Słupek E, Makoś P, Cieśliński H, Kamiński M (2020) Influence of alkaline and oxidative pre-treatment of waste corn cobs on biohydrogen generation efficiency via dark fermentation. Biomass Bioenergy 141:105691
Kumar JCR, Majid MA (2020) Renewable energy for sustainable development in India: current status, future prospects, challenges, employment, and investment opportunities. Energy Sustain Soci 10(2):1–36
Kumar A, Samadder SJE (2020) Performance evaluation of anaerobic digestion technology for energy recovery from organic fraction of municipal solid waste: a review. Energy 197:117253
Kumar A, Saini K, Bhaskar T (2020a) Hydochar and biochar: production, physicochemical properties and techno-economic analysis. Bioresour Technol 310:123442
Kumar MD, Kannah RY, Kumar G, Sivashanmugam P, Banu JR (2020b) A novel energetically efficient combinative microwave pretreatment for achieving profitable hydrogen production from marine macro algae (Ulva reticulate). Bioresour Technol 301:122759
Kundu R, Ramasubramanian V, Neeli ST, Ramsurn H (2021) Catalytic pyrolysis of methane to hydrogen over carbon (from cellulose biochar) encapsulated iron nanoparticles. Energy Fuels 35(16):13523–13533
Kusworo TD, Widayat W, Mahadita AF, Firizqina D, Utomo DP (2020) Bio-oil and fuel gas production from agricultural waste via pyrolysis: a comparative study of oil palm empty fruit bunches (OPEFB) and rice husk. Period Polytech Chem Eng 64(2):179–191
Kwak J-H, Islam MS, Wang S, Messele SA, Naeth MA, El-Din MG, Chang SXJC (2019) Biochar properties and lead (II) adsorption capacity depend on feedstock type, pyrolysis temperature, and steam activation. Chemosphere 231:393–404
Kwong K, Marek EJ (2021) Combustion of biomass in fluidized beds: a review of key phenomena and future perspectives. Energy Fuels 35(20):16303–16334
Lachos-Perez D, Torres-Mayanga PC, Abaide ER, Zabot GL, De Castilhos FJBT (2022) Hydrothermal carbonization and liquefaction: differences, progress, challenges, and opportunities. Bioresour Technol 343:126084
Las-Heras-Casas J, López-Ochoa LM, Paredes-Sánchez JP, López-González LM (2018) Implementation of biomass boilers for heating and domestic hot water in multi-family buildings in Spain: energy, environmental, and economic assessment. J Clean Prod 176:590–603
Li LZ, Chen J, Yan KS, Qin XM, Feng T, Wang JW, Wang FM (2018a) Methane dry reforming with microwave heating over carbon-based catalyst obtained by agriculture residues pyrolysis. J CO2 Utilization 28:41–49
Li M, Zhang H, Xiao TF, Wang SD, Zhang BP, Chen DY, Su MH, Tang JF (2018b) Low-cost biochar derived from corncob as oxygen reduction catalyst in air cathode microbial fuel cells. Electrochim Acta 283:780–788
Li XZ, Chen ZJ, Fan XC, Cheng ZJ (2018c) Hydropower development situation and prospects in China. Renew Sustain Energy Rev 82(1):232–239
Li M, Ci S, Ding Y, Wen Z (2019) Almond shell derived porous carbon for high-performance anode of microbial fuel cells. Sustain Energy Fuels 3:3415–3421
Li WM, He L, Cheng C, Cao GL, Ren NQ (2020a) Effects of biochar on ethanol-type and butyrate-type fermentative hydrogen productions. Bioresour Technol 306:123088
Li Y, Zhu Q, Ding P, You S, Zhang Q, Jiang, H.J.I.J.o.H.E. (2020b) Effects of Fe0 and Ni0 nanoparticles on hydrogen production from cotton stalk hydrolysate using Klebsiella sp. WL1316: evaluation of size and concentration of the nanoparticles. Int J Hydrog Energy 45(11):6243–6253
Li P, Shi X, Wang X, Song J, Fang S, Bai J, Zhang G, Chang C, Pang S (2021a) Bio-oil from biomass fast pyrolysis: yields, related properties and energy consumption analysis of the pyrolysis system. J Clean Prod 328:129613
Li X, Lu Z, Chen J, Chen X, Jiang Y, Jian J, Yao SJF (2021b) Effect of oxidative torrefaction on high temperature combustion process of wood sphere. Fuel 286:119379
Li B, Mbeugang CFM, Huang Y, Liu D, Wang Q, Zhang SJE (2022) A review of CaO based catalysts for tar removal during biomass gasification. Energy. https://doi.org/10.1016/j.energy.2022.123172
Lin BQ, Ge JM (2020) To harvest or not to harvest? Forest management as a trade-off between bioenergy production and carbon sink. J Clean Prod 268:122219
Lin YC, Amesho KTT, Chen CE, Cheng PC, Chou FC (2020) A cleaner process for green biodiesel synthesis from waste cooking oil using recycled waste oyster shells as a sustainable base heterogeneous catalyst under the microwave heating system. Sustain Chem Pharm 17:100310
Liu JX (2019) China’s renewable energy law and policy: a critical review. Renew Sustain Energy Rev 99:212–219
Liu HC, Huang YQ, Yuan HY, Yin XL, Wu CZ (2018) Life cycle assessment of bifuels in china: Status and challenges. Renew Sustain Energy Rev 97:301–322
Ližbetin J, Hlatká M, Bartuška L (2018) Issues concerning declared energy consumption and greenhouse gas emissions of FAME biofuels. Sustainability 10(9):3025
Low YW, Yee KF (2021) A review on lignocellulosic biomass waste into biochar-derived catalyst: current conversion techniques, sustainable applications and challenges. Biomass Bioenerg 154:106245
Lu JS, Chang YJ, Poon CS, Lee DJ (2020) Slow pyrolysis of municipal solid waste (MSW): a review. Bioresour Technol 312:123615
Lu J, Jia Z, Wang P, Yang X, Lin P, Ren L, Farghali M (2022) Restoration of acidified dry anaerobic digestion of food waste: bioaugmentation of butyric acid-resistant microbes. J Environ Chem Eng 10(1):106935
Luo T, Huang H, Mei Z, Shen F, Ge Y, Hu G, Meng X (2019) Hydrothermal pretreatment of rice straw at relatively lower temperature to improve biogas production via anaerobic digestion. Chin Chem Lett 30(6):1219–1223
Ma Y, Liu Y (2019) Turning food waste to energy and resources towards a great environmental and economic sustainability: an innovative integrated biological approach. Biotechnol Adv. https://doi.org/10.1016/j.biotechadv.2019.06.013
Malico I, Nepomuceno Pereira R, Gonçalves AC, Sousa AM (2019) Current status and future perspectives for energy production from solid biomass in the European industry. Renew Sustain Energy Rev 112:960–977
Mansur FZ, Faizal CKM, Monir MU, Samad NAFA, Atnaw SM, Sulaiman SA (2020) Co-gasification between coal/sawdust and coal/wood pellet: a parametric study using response surface methodology. Int J Hydrog Energy 45(32):15963–15976
Marzorati S, Goglio A, Fest-Santini S, Mombelli D, Villa F, Cristiani P, Schievano A (2018) Air-breathing bio-cathodes based on electro-active biochar from pyrolysis of Giant Cane stalks. Int J Hydrog Energy. https://doi.org/10.1016/j.ijhydene.2018.07.167
Masoudi M, Rahimnejad M, Mashkour M (2020) Fabrication of anode electrode by a novel acrylic based graphite paint on stainless steel mesh and investigating biofilm effect on electrochemical behavior of anode in a single chamber microbial fuel cell. Electrochim Acta 344:136168
Mathimani T, Baldinelli A, Rajendran K, Prabakar D, Matheswaran M, van Leeuwen RP, Pugazhendhi A (2019) Review on cultivation and thermochemical conversion of microalgae to fuels and chemicals: process evaluation and knowledge gaps. J Clean Prod 208:1053–1064
Megía PJ, Vizcaíno AJ, Calles JA, Carrero A (2021) Hydrogen production technologies: from fossil fuels toward renewable sources. A mini review. Energy Fuels 35(20):16403–16415
Mishra S, Upadhyay RK (2021) Review on biomass gasification: gasifiers, gasifying mediums, and operational parameters. Mater Sci Energy Technol 4:329–340
Mishra P, Krishnan S, Rana S, Singh L, Sakinah M, Ab Wahid Z (2019) Outlook of fermentative hydrogen production techniques: an overview of dark, photo and integrated dark-photo fermentative approach to biomass. Energ Strat Rev 24:27–37
Mohamed HH (2022) Green processes and sustainable materials for renewable energy production via water splitting. In: Cheong KY, Apblett A (eds) Sustainable materials and green processing for energy conversion. Elsevier, Amsterdam
Mohamed RM, Kadry GA, Abdel-Samad HA, Awad ME (2020) High operative heterogeneous catalyst in biodiesel production from waste cooking oil. Egypt J Pet 29:59–65
Mohammadi Moradian J, Wang SM, Ali A, Liu JY, Mi JL, Wang HC (2022) Biomass-derived carbon anode for high-performance microbial fuel cells. Catalysts 12:894
Munoz-Cupa C, Hu YL, Xu CB, Bassi A (2021) An overview of microbial fuel cell usage in wastewater treatment, resource recovery and energy production. Sci Total Environ 754:142429
Muthudineshkumar R, Anand R (2019) Anaerobic digestion of various feedstocks for second-generation biofuel production. In: Azad K (ed) Advances in eco-fuels for a sustainable environment. Woodhead Publishing, Sawston, pp 157–185
Mwale S, Munyati MO, Nyirenda J (2020) Preparation, characterization, and optimization of a porous polyaniline-copper anode microbial fuel cell. J Solid State Electrochem 25:639–650
Nastro RA, Flagiello F, Nicandro S, Gambino E, Falcucci G, Chandrasekhar K (2021) Use of biochar-based cathodes and increase in the electron flow by Pseudomonas aeruginosa to improve waste treatment in microbial fuel cells. Processes 9:1941
Naveenkumar M, Senthilkumar K (2021) Microbial fuel cell for harvesting bio-energy from tannery effluent using metal mixed biochar electrodes. Biomass Bioenerg 149:106082
Nazir MS, Mahdi AJ, Bilal M, Sohail HM, Ali N, Iqbal HM (2019) Environmental impact and pollution-related challenges of renewable wind energy paradigm–a review. Sci Total Environ 683:436–444
Nzediegwu C, Arshad M, Ulah A, Naeth MA, Chang SX (2021) Fuel, thermal and surface properties of microwave-pyrolyzed biochars depend on feedstock type and pyrolysis temperature. Bioresour Technol 320:124282
Organization for Economic Cooperation and Development (2022) Municipal waste, generation and treatment
Osman AI, Mehta N, Elgarahy AM, Al-Hinai A, Al-Muhtaseb AAH, Rooney DW (2021) Conversion of biomass to biofuels and life cycle assessment: a review. Environ Chem Lett 19(6):4075–4118
Osman AI, Chen L, Yang M, Msigwa G, Farghali M, Fawzy S, Rooney DW, Yap P-S (2022a) Cost, environmental impact, and resilience of renewable energy under a changing climate: a review. Environ Chem Lett 21:741–764. https://doi.org/10.1007/s10311-022-01532-8
Osman AI, Fawzy S, Farghali M, El-Azazy M, Elgarahy AM, Fahim RA, Maksoud MIAA, Ajlan AA, Yousry M, Saleem Y, Rooney DW (2022b) Biochar for agronomy, animal farming, anaerobic digestion, composting, water treatment, soil remediation, construction, energy storage, and carbon sequestration: a review. Environ Chem Lett 20(4):2385–2485
Osman AI, Farghali M, Ihara I, Elgarahy AM, Ayyad A, Mehta N, Ng KH, Abd El-Monaem EM, Eltaweil AS, Hosny M, Hamed SM, Fawzy S, Yap P-S, Rooney DW (2023) Materials, fuels, upgrading, economy, and life cycle assessment of the pyrolysis of algal and lignocellulosic biomass: a review. Environ Chem Lett 21:1419–1476.https://doi.org/10.1007/s10311-023-01573-7
Palanisamy G, Jung HY, Sadhasivam T, Kurkuri MD, Kim SC, Roh SH (2019) A comprehensive review on microbial fuel cell technologies: processes, utilization, and advanced developments in electrodes and membranes. J Clean Prod 221:598–621
Panahi HKS, Dehhaghi M, Ok YS, Nizami A-S, Khoshnevisan B, Mussatto SI, Aghbashlo M, Tabatabaei M, Lam SS (2020) A comprehensive review of engineered biochar: production, characteristics, and environmental applications. J Clean Prod 270(122462):62
Pata UK, Kumar A (2021) The influence of hydropower and coal consumption on greenhouse gas emissions: a comparison between China and India. Water 13(10):1387
Patel S, Kundu S, Halder P, Marzbali MH, Chiang K, Surapaneni A, Shah K (2020) Production of hydrogen by catalytic methane decomposition using biochar and activated char produced from biosolids pyrolysis. Int J Hydrog Energy 45(55):29978–29992
Patwardhan SB, Pandit S, Gupta PK, Kumar Jha N, Rawat J, Joshi HC, Priya K, Gupta M, Lahiri D, Nag M, Kumar Thakur V, Kumar Kesari K (2022) Recent advances in the application of biochar in microbial electrochemical cells. Fuel 311:122501
Peng X, Jiang Y, Chen Z, Osman AI, Farghali M, Rooney DW, Yap P-S (2023) Recycling municipal, agricultural and industrial waste into energy, fertilizers, food and construction materials, and economic feasibility: a review. Environ Chem Lett 21(2):765–801
Pergola M, Rita A, Tortora A, Castellaneta M, Borghetti M, De Sergio Franchi A, Lapolla A, Moretti N, Pecora G, Pierangeli D, Todaro L, Ripullone F (2020) Identification of suitable areas for biomass power plant construction through environmental impact assessment of forest harvesting residues transportation. Energies 13(11):2699
Perrot J-F, Subiantoro A (2018) Municipal waste management strategy review and waste-to-energy potentials in New Zealand. Sustainability 10(9):3114
Phuttaro C, Sawatdeenarunat C, Surendra K, Boonsawang P, Chaiprapat S, Khanal SK (2019) Anaerobic digestion of hydrothermally-pretreated lignocellulosic biomass: influence of pretreatment temperatures, inhibitors and soluble organics on methane yield. Bioresour Technol 284:128–138
Pischke EC, Solomon B, Wellstead A, Acevedo A, Eastmond A, De Oliveira F, Coelho S, Lucon O (2019) From Kyoto to Paris: measuring renewable energy policy regimes in Argentina, Brazil, Canada, Mexico and the United States. Energy Res Soc Sci 50:82–91
Pisciotta M, Pilorgé H, Feldmann J, Jacobson R, Davids J, Swett S, Sasso Z, Wilcox J (2022) Current state of industrial heating and opportunities for decarbonization. Prog Energy Combust Sci 91:100982
Qian X, Shen G, Wang Z, Zhang X, Chen X, Tang Z, Lei Z, Zhang Z (2019) Enhancement of high solid anaerobic co-digestion of swine manure with rice straw pretreated by microwave and alkaline. Bioresour Technol Rep 7:100208
Qiu L, Deng YF, Wang F, Davaritouchaee M, Yao YQ (2019) A review on biochar-mediated anaerobic digestion with enhanced methane recovery. Renew Sustain Energy Rev 115:109373
Rago YP, Collard F-X, Görgens JF, Surroop D, Mohee R (2020) Torrefaction of biomass and plastic from municipal solid waste streams and their blends: evaluation of interactive effects. Fuel 277:118089
Rahman MM (2020) Environmental degradation: the role of electricity consumption, economic growth and globalisation. J Environ Manage 253:109742
Rahman WU, Yahya SM, Khan ZA, Khan NA, Halder G, Dhawane SH (2021) Valorization of waste chicken egg shells towards synthesis of heterogeneous catalyst for biodiesel production: optimization and statistical analysis. Environ Technol Innov 22:101460
Rajendran K, Mahapatra D, Venkatraman AV, Muthuswamy S, Pugazhendhi A (2020) Advancing anaerobic digestion through two-stage processes: current developments and future trends. Renew Sustain Energy Rev 123:109746
Rambabu K, Bharath G, Banat F, Hai A, Show PL (2021) Ferric oxide/date seed activated carbon nanocomposites mediated dark fermentation of date fruit wastes for enriched biohydrogen production. Int J Hydrog Energy 46(31):16631–16643
Ramya M, Harsha Vardhan K, Senthil Kumar P (2022) Metal mixed biochar electrodes for the generation of electricity with high power density in microbial fuel cell. Sustain Energy Technol Assess 53(B):102549
Ravindran B, Karmegam N, Yuvaraj A, Thangaraj R, Chang S, Zhang Z, Awasthi MK (2021) Cleaner production of agriculturally valuable benignant materials from industry generated bio-wastes: a review. Bioresour Technol 320:124281
Rehfeldt M, Worrell E, Eichhammer W, Fleiter T (2020) A review of the emission reduction potential of fuel switch towards biomass and electricity in European basic materials industry until 2030. Renew Sustain Energy Rev 120:109672
Ren J, Cao J-P, Zhao X-Y, Yang F-L, Wei X-Y (2019) Recent advances in syngas production from biomass catalytic gasification: a critical review on reactors, catalysts, catalytic mechanisms and mathematical models. Renew Sustain Energy Rev 116:109426
Ren J, Liu Y-L, Zhao X-Y, Cao JP (2020) Methanation of syngas from biomass gasification: an overview. Int J Hydrog Energy 45(7):4223–4243
Rodríguez-Valderrama S, Escamilla-Alvarado C, Magnin J-P, Rivas-García P, Valdez-Vazquez I, Ríos-Leal E (2020) Batch biohydrogen production from dilute acid hydrolyzates of fruits-and-vegetables wastes and corn stover as co-substrates. Biomass Bioenerg 140:105666
Saadi M, Pézard J, Haddour N, Erouel M, Vogel TM, Khirouni K (2020) Stainless steel coated with carbon nanofiber/PDMS composite as anodes in microbial fuel cells. Mater Res Express 7(2):025504
Sahu O (2021) Characterisation and utilization of heterogeneous catalyst from waste rice-straw for biodiesel conversion. Fuel. https://doi.org/10.1016/j.fuel.2020.119543
Sajid M, Raheem A, Ullah N, Asim M, Rehman MSU, Ali N (2022) Gasification of municipal solid waste: progress, challenges, and prospects. Renew Sustain Energy Rev 168:112815
Saravanan A, Kumar PS, Aron NS, Jeevanantham S, Karishma S, Yaashikaa P, Chew KW, Show PL (2022a) A review on bioconversion processes for hydrogen production from agro-industrial residues. Int J Hydrog Energy 47(88):37302–37320
Saravanan A, Kumar PS, Jeevanantham S, Karishma S, Vo DV (2022b) Recent advances and sustainable development of biofuels production from lignocellulosic biomass. Bioresour Technol 344:126203
Sarkar JK, Wang Q (2020) Different pyrolysis process conditions of South Asian waste coconut shell and characterization of gas, bio-char, and bio-oil. Energies 13(8):1970
Sarto S, Hildayati R, Syaichurrozi I (2019) Effect of chemical pretreatment using sulfuric acid on biogas production from water hyacinth and kinetics. Renew Energy 132:335–350
Shaah MAH, Hossain MS, Salem Allafi FA, Alsaedi A, Ismail N, Ab Kadir MO, Idayu Ahmad M (2021) A review on non-edible oil as a potential feedstock for biodiesel: physicochemical properties and production technologies. RSC Adv 11:25018–25037
Shaikh R, Rizvi A, Quraishi M, Pandit S, Mathuriya AS, Gupta PK, Singh J, Prasad R (2020) Bioelectricity production using plant-microbial fuel cell: present state of art. S Afr J Bot. https://doi.org/10.1016/j.sajb.2020.09.025
Shimahata A, Mohamed IMA, Iwasaki M, Lu J, Ihara I, Umetsu K (2022) Integrating anaerobic digestion with hydrothermal pretreatment for bioenergy production: waste valorization of plastic containing food waste and rice husk. Biochem Eng J 186:108546
Sillero L, Solera R, Pérez M (2022) Effect of the hydraulic retention time on the acidogenic fermentation of sewage sludge, wine vinasse and poultry manure for biohydrogen production. Biomass Bioenerg 167:106643
Sim Y-B, Jung J-H, Baik J-H, Park J-H, Kumar G, Banu JR, Kim SH (2021) Dynamic membrane bioreactor for high rate continuous biohydrogen production from algal biomass. Bioresour Technol 340:125562
Sindhu R, Binod P, Pandey A, Ankaram S, Duan Y, Awasthi MK (2019) Biofuel production from biomass: toward sustainable development. In: Current development in biotechnology and bioengineering, pp 79–92
Singh R, Hans M, Kumar S, Yadav YK (2023) Thermophilic anaerobic digestion: an advancement towards enhanced biogas production from lignocellulosic biomass. Sustainability 15(3):1859
Sivabalan K, Hassan S, Ya H, Pasupuleti J (2020) A review on the characteristic of biomass and classification of bioenergy through direct combustion and gasification as an alternative power supply. In: Journal of physics: conference series 1831:28–29
Sivabalan K, Hassan S, Ya H, Pasupuleti J (2021) A review on the characteristic of biomass and classification of bioenergy through direct combustion and gasification as an alternative power supply. In: Journal of physics: conference series. IOP Publishing, pp 012033
Siwal SS, Zhang Q, Sun C, Thakur S, Gupta VK, Thakur VK (2020) Energy production from steam gasification processes and parameters that contemplate in biomass gasifier–a review. Bioresour Technol 297:122481
Slate AJ, Whitehead KA, Brownson DAC, Banks CE (2019) Microbial fuel cells: an overview of current technology. Renew Sustain Energy Rev 101:60–81
Sonu K, Sogani M, Syed Z, Dongre A, Sharma G (2020) Effect of corncob derived biochar on microbial electroremediation of dye wastewater and bioenergy generation. Chem Sel. https://doi.org/10.1002/slct.202002652
Sugiarto Y, Sunyoto NMS, Zhu MM, Jones I, Zhang D (2021) Effect of biochar in enhancing hydrogen production by mesophilic anaerobic digestion of food wastes: the role of minerals. Int J Hydrog Energy 46(5):3695–3703
SundarRajan P, Gopinath KP, Arun J, GracePavithra K, Pavendan K, AdithyaJoseph A (2020) An insight into carbon balance of product streams from hydrothermal liquefaction of Scenedesmus abundans biomass. Renew Energy 151:79–87
Tan YH, Abdullah MO, Kansedo J, Mubarak NM, San Chan Y, Nolasco-Hipolito C (2019) Biodiesel production from used cooking oil using green solid catalyst derived from calcined fusion waste chicken and fish bones. Renew Energy 139:696–706
Tan M, Li H, Huang Z, Wang Z, Xiong R, Jiang S, Zhang J, Wu Z, Li C, Luo L (2021) Comparison of atmospheric and gas-pressurized oxidative torrefaction of heavy-metal-polluted rice straw. J Clean Prod 283:124636
Tańczuk M, Junga R, Werle S, Chabiński M (2019) Experimental analysis of the fixed bed gasification process of the mixtures of the chicken manure with biomass. Renew Energy 136:1055–1063
Tanyaket T, Onsree T, Tippayawong N, Baratieri MJ (2020) Effect of oxidative torrefaction on characteristics of treated corncob pellets.中國機械工程學刊 41(1):65–73
Taufiq A, Arista S, Sutrisno B, Hidayat A (2020) Biodiesel synthesis over biochar-based catalyst from Sengon (Paraserianthes falcataria L. Nielsen) sawdust using palm fatty acid distillate as low-cost feedstock. In: IOP conference series: materials science and engineering, vol 778. IOP Publishing, Kuala Lumpur, pp 012111
Tezer Ö, Karabağ N, Öngen A, Çolpan CÖ, Ayol A (2022) Biomass gasification for sustainable energy production: a review. Int J Hydrog Energy. https://doi.org/10.1016/j.ijhydene.2022.02.158
Theuerl S, Klang J, Prochnow A (2019) Process disturbances in agricultural biogas production—causes, mechanisms and effects on the biogas microbiome: a review. Energies 12(3):365
Tian Y, Wang F, Djandja JO, Zhang S-L, Xu Y-P, Duan PG (2020) Hydrothermal liquefaction of crop straws: effect of feedstock composition. Fuel 265:116946
Tomczyk A, Sokołowska Z, Boguta P (2020) Biochar physicochemical properties: pyrolysis temperature and feedstock kind effects. Rev Environ Sci Bio Technol 19:191–215
Tong S, Sun Y, Li X, Hu Z, Worasuwannarak N, Liu H, Hu H, Luo G, Yao H (2021) Gas-pressurized torrefaction of biomass wastes: co-gasification of gas-pressurized torrefied biomass with coal. Bioresour Technol 321:124505
Tripathi B, Pandit S, Sharma A, Chauhan S, Mathuriya AS, Dikshit PK, Gupta PK, Singh RC, Sahni M, Pant K, Singh S (2022) Modification of graphite sheet anode with iron (II, III) oxide-carbon dots for enhancing the performance of microbial fuel cell. Catalysts 12(9):1040
Tshemese Z, Deenadayalu N, Linganiso LZ, Chetty M (2023) An overview of biogas production from anaerobic digestion and the possibility of using sugarcane wastewater and municipal solid waste in a South African context. Appl Syst Innov 6(1):13
United Nations (2022) World population prospects 2022: summary of results. New York, NY: United Nations
Veers P, Dykes K, Lantz E, Barth S, Bottasso CL, Carlson O, Clifton A, Green J, Green P, Holttinen H, Laird D, Lehtomäki V, Lundquist JK, Manwell J, Marquis M, Meneveau C, Moriarty P, Munduate X, Muskulus M, Naughton J, Pao L, Paquette J, Peinke J, Robertson A, Sanz Rodrigo J, Sempreviva AM, Smith JC, Tuohy A, Wiser R (2019) Grand challenges in the science of wind energy. Science 366(6464):eaau2027
Veldkamp E, Schmidt M, Powers JS, Corre MD (2020) Deforestation and reforestation impacts on soils in the tropics. Nat Rev Earth Environ 1:590–605
Voropai N, Podkovalnikov S, Savelyev V, Palamarchuk S (2019) Hydropower development in Eastern Russia in the context of interstate cooperation: current state and prospects. Energy Syst Res 2(5):43–51
Wang GJ, Li Q, Dzakpasu M, Gao XG, Yuwen CS, Wang XCC (2018) Impacts of different biochar types on hydrogen production promotion during fermentative co-digestion of food wastes and dewatered sewage sludge. Waste Manage 80:73–80
Wang Z, Lim CJ, Grace JRJE (2019) A comprehensive study of sawdust torrefaction in a dual-compartment slot-rectangular spouted bed reactor. Energy 189:116306
Wang C, Zhang X, Liu Q, Zhang Q, Chen L, Ma LJFPT (2020a) A review of conversion of lignocellulose biomass to liquid transport fuels by integrated refining strategies. Fuel Process Technol 208:106485
Wang D, Jiang PK, Zhang HB, Yuan WQ (2020b) Biochar production and applications in argo and foresty systems: a review. Sci Total Environ 723:137775
Wang G, Dai Y, Yang H, Xiong Q, Wang K, Zhou J, Li Y, Wang S (2020c) A review of recent advances in biomass pyrolysis. Energy Fuels 34(12):15557–15578
Wang J, Zhang SL, Zhang QJ (2021) The relationship of renewable energy consumption to financial development and economic growth in China. Renew Energy 170:897–904
Wang Y, Akbarzadeh A, Chong L, Du J, Tahir N, Awasthi MK (2022) Catalytic pyrolysis of lignocellulosic biomass for bio-oil production: a review. Chemosphere 297:134181
World Bioenergy Association (2021) Global Bioenergy Statistics 2021. Stockholm, Swedem, World Bioenergy Association
Wu JW, Dong LL, Zhou CS, Liu BF, Xing DF, Feng LP, Wu XK, Wang Q, Cao G (2019) Enhanced butanol-hydrogen coproduction by Clostridium beijerinckii with biochar as cell’s carrier. Bioresour Technol 294:122141
Wu X, Li CH, Shao L, Meng J, Zhang LX, Chen GQ (2021) Is solar power renewable and carbon-neutral: evidence from a pilot solar tower plant in China under a systems view. Renew Sustain Energy Rev 138:110655
Xu Y-H, Li M-FJBT (2021) Hydrothermal liquefaction of lignocellulose for value-added products: mechanism, parameter and production application. Bioresour Technol 342:126035
Xu XF, Wei ZF, Ji Q, Wang CL, Gao GW (2019) Global renewable energy development: influencing factors, trend predictions and countermeasures. Resour Policy 63:101470
Xu MY, Kang Y, Jiang LL, Jlang L, Tremblay PL, Zhang T (2022) The one-step hydrothermal synthesis of CdS nanorods modified with carbonized leaves from Japanese raisin trees for photocatalytic hydrogen evolution. Int J Hydrog Energy 47:15516–15527
Xue Y, Zhou S, Leng E, Cui C, Zhou Z, Peng Y (2021) Comprehensive utilization of agricultural wastes by combined wet torrefaction and pyrolysis. J Anal Appl Pyrolysis 160:105358
Yaashikaa PR, Senthil Kumar P, Varjani S, Saravanan A (2020) A critical review on the biochar production techniques, characterization, stability and applications for circular bioeconomy. Biotechnol Rep 28:e00570
Yang W, Chen S (2020) Biomass-derived carbon for electrode fabrication in microbial fuel cells: a review. Ind Eng Chem Res 59(14):6391–6404
Yang G, Wang JL (2019) Synergistic enhancement of biohydrogen production from grass fermentation using biochar combined with zero-valent iron nanoparticles. Fuel 251:420–427
Yang CC, Chen MJ, Qian YJ, Zhang L, Lu M, Xie XJ, Huang L, Huang W (2018) Packed anode derived from cocklebur fruit for improving long-term performance of microbial fuel cells. Sci China Mater 62(5):645–652
Yang W, Chata G, Zhang YD, Peng Y, Lu JE, Wang N, Mercado R, Li J, Chen SW (2019) Graphene oxide-supported zinc cobalt oxides as effective cathode catalysts for microbial fuel cell: high catalytic activity and inhibition of biofilm formation. Nano Energy 57:811–819
Yang Z, Yang RM, Dong GX, Xiang M, Hui J, Ou JF, Qin HF (2021) Biochar nanocomposite derived from watermelon peels for electrocatalytic hydrogen production. ACS Omega 6(3):2066–2073
Yao ZY, You SM, Ge TS, Wang CH (2018) Biomass gasification for syngas and biochar co-production: energy application and economic evaluation. Appl Energy 209:43–55
Yaqoob AA, Mohamad Ibrahim MN, Rodríguez-Couto S (2020) Development and modification of materials to build cost-effective anodes for microbial fuel cells (MFCs): an overview. Biochem Eng J 164:107779
Yaqoob AA, Mohamad Ibrahim MN, Guerrero-Barajas C (2021a) Modern trend of anodes in microbial fuel cells (MFCs): an overview. Environ Technol Innov 23:101579
Yaqoob AA, Mohamad Ibrahim MN, Umar K (2021b) Biomass-derived composite anode electrode: synthesis, characterizations, and application in microbial fuel cells (MFCs). J Environ Chem Eng 9(5):106111
Yaqoob AA, Mohamad Ibrahim MN, Yaakop AS, Umar K, Ahmad A (2021c) Modified graphene oxide anode: a bioinspired waste material for bioremediation of Pb2+ with energy generation through microbial fuel cells. Chem Eng J 417:128052
Yaşar F (2019) Biodiesel production via waste eggshell as a low-cost heterogeneous catalyst: its effects on some critical fuel properties and comparison with CaO. Fuel 255:115828
Ye B, Zhang R, Cao J, Lei K, Liu D (2020) The study of co-combustion characteristics of coal and microalgae by single particle combustion and TGA methods. J Energy Inst 93(2):508–517
Yek PN, Mahari WA, Kong SH, Foong SY, Peng W, Ting H, Liew RK, Xia C, Sonne C, Tabatabaei M, Almomani F (2022) Pilot-scale co-processing of lignocellulosic biomass, algae, shellfish waste via thermochemical approach: recent progress and future directions. Bioresour Technol 347:126687
Yin Y, Hu J, Wang J (2019) Fermentative hydrogen production from macroalgae Laminaria japonica pretreated by microwave irradiation. Intl J Hydrog Energy 44(21):10398–10406
Ying Z, Geng Z, Zheng X, Dou B, Cui G (2021) Enhancing biochar oxidation reaction with the mediator of Fe2+/Fe3+ or NO2-/NO3- for efficient hydrogen production through biochar-assisted water electrolysis. Energy Convers Manage 244:114523
Ying Z, Geng Z, Zheng XY, Dou BL, Cui GM (2022) Improving water electrolysis assisted by anodic biochar oxidation for clean hydrogen production. Energy 238:121793
Yogalakshmi K, Sivashanmugam P, Kavitha S, Kannah Y, Varjani S, AdishKumar S, Kumar GJC (2022) Lignocellulosic biomass-based pyrolysis: a comprehensive review. Chemosphere 286:131824
Yuan T, He WJ, Yin GJ, Xu SA (2020) Comparison of bio-chars formation derived from fast and slow pyrolysis of walnut shell. Fuel 261:116450
Zha ZT, Zhang Z, Xiang P, Zhu HY, Zhou BM, Sun ZL, Zhou S (2021) One-step preparation of eggplant-derived hierarchical porous graphitic biochar as efficient oxygen reduction catalyst in microbial fuel cells. RSC Adv 11:1077
Zhang C, Ho S-H, Chen W-H, Fu Y, Chang J-S, Bi XJAE (2019a) Oxidative torrefaction of biomass nutshells: evaluations of energy efficiency as well as biochar transportation and storage. Appl Energy 235:428–441
Zhang C, Wang C, Cao G, Chen W-H, Ho S-HJF (2019b) Comparison and characterization of property variation of microalgal biomass with non-oxidative and oxidative torrefaction. Fuel 246:375–385
Zhang L, Li Y, Liu X, Ren N, Ding J (2019c) Lignocellulosic hydrogen production using dark fermentation by Clostridium lentocellum strain Cel10 newly isolated from Ailuropoda melanoleuca excrement. RSC Adv 9(20):11179–11185
Zhang Y, Cui Y, Chen P, Liu S, Zhou N, Ding K, Fan L, Peng P, Min M, Cheng Y (2019d) Gasification technologies and their energy potentials. In: Taherzadeh M, Bolton K, Wong J, Pandey A (eds) Sustainable resource recovery and zero waste approaches. Elsevier, Amsterdam, pp 193–206
Zhang S, Zhou S, Yang X, Xi W, Zheng K, Chu C, Ju M, Liu L (2020a) Effect of operating parameters on hydrothermal liquefaction of corn straw and its life cycle assessment. Environ Sci Pollut Res 27:6362–6374
Zhang X, Geng Y, Shao S, Wilson J, Song XQ, You W (2020b) China’s non-fossil energy development and its 2030 CO2 reduction targets: the role of urbanization. Appl Energy 261:114353
Zhang C, Ho S-H, Chen W-H, Wang R, Show P-L, Ong HC (2021a) Oxidative torrefaction performance of microalga Nannochloropsis Oceanica towards an upgraded microalgal solid biofuel. J Biotechnol 338:81–90
Zhang C, Ho S-H, Chen W-H, Wang RJE (2021b) Comparative indexes, fuel characterization and thermogravimetric-Fourier transform infrared spectrometer-mass spectrogram (TG-FTIR-MS) analysis of microalga Nannochloropsis Oceanica under oxidative and inert torrefaction. Energy 230:120824
Zhang J, Cui YX, Zhang TY, Hu Q, Tong YW, He YL, Dai YJ, Peng YH (2021c) Food waste treating by biochar-assisted high-solid anaerobic digestion coupled with steam gasification: enhanced bioenergy generation and porous biochar production. Biores Technol 331:125051
Zhang L, Wang Z, Ma J, Kong W, Yuan P, Sun R, Shen BJF (2022) Analysis of functionality distribution and microstructural characteristics of upgraded rice husk after undergoing non-oxidative and oxidative torrefaction. Fuel 310:122477
Zhao C, Lv PM, Yang LM, Xing SY, Luo W, Wang ZM (2018) Biodiesel synthesis over biochar-based catalyst from biomass waste pomelo peel. Energy Convers Manage 160:477–485
Zhao L, Chen C, Ren HY, Wu JT, Meng J, Nan J, Cao GL, Yang SS, Ren NQ (2019a) Feasibility of enhancing hydrogen production from cornstalk hydrolysate anaerobic fermentation by RCPH-biochar. Bioresour Technol 297:122505
Zhao L, Zhang J, Zhao W, Zang L (2019b) Improved fermentative hydrogen production with the addition of calcium lignosulfonate-derived biochar. Energy Fuels 33:7406–7414. https://doi.org/10.1021/acs.energyfuels.9b01380
Zhao L, Wang ZH, Ren HY, Chen C, Nan J, Cao GL, Yang SS, Ren NQ (2021) Residue cornstalk derived biochar promotes direct bio-hydrogen production from anaerobic fermentation of cornstalk. Bioresour Technol 320:124338
Zhao A, Liu S, Yao J, Huang F, He Z, Liu JJBT (2022a) Characteristics of bio-oil and biochar from cotton stalk pyrolysis: Effects of torrefaction temperature and duration in an ammonia environment. Bioresour Technol 343:126145
Zhao S, Zhang LJ, Hu HQ, Jin LJ (2022b) Catalytic decomposition of methane over enteromorpha prolifera-based hierarchical porous biochar. Int J Hydrog Energy. https://doi.org/10.1016/j.ijhydene.2022.10.215
Zheng HY, Song ML, Shen ZY (2021) The evolution of renewable energy and its impact on carbon reduction in China. Energy 237:121639
Zhong K, Li M, Yang Y, Zhang H, Zhang B, Tang J, Yan J, Su MH, Yang Z (2019) Nitrogen-doped biochar derived from watermelon rind as oxygen reduction catalyst in air cathode microbial fuel cells. Appl Energy 242:516–525
Zhou N, Zhou J, Dai L, Guo F, Wang Y, Li H, Deng W, Lei H, Chen P, Liu YJBT (2020) Syngas production from biomass pyrolysis in a continuous microwave assisted pyrolysis system. Bioresour Technol 314:123756
Zhu HL, Zhang YS, Materazzi M, Aranda G, Brett DJ, Shearing PR, Manos GJFPT (2019) Co-gasification of beech-wood and polyethylene in a fluidized-bed reactor. Fuel Process Technol 190:29–37
Ziara RM, Miller DN, Subbiah J, Dvorak BI (2019) Lactate wastewater dark fermentation: the effect of temperature and initial pH on biohydrogen production and microbial community. Int J Hydrog Energy 44(2):661–673
Zulqarnain, Mohd Yusoff MH, Ayoub M, Ramzan N, Nazir MH, Zahid I, Abbas N, Elboughdiri N, Mirza CR, Butt TA (2021) Overview of feedstocks for sustainable biodiesel production and implementation of the biodiesel program in Pakistan. ACS Omega 6(29):19099–19114
Acknowledgements
Dr. Ahmed I. Osman and Prof. David W. Rooney wish to acknowledge the support of The Bryden Centre project (Project ID VA5048), which was awarded by The European Union’s INTERREG VA Programme, managed by the Special EU Programmes Body (SEUPB), with match funding provided by the Department for the Economy in Northern Ireland and the Department of Business, Enterprise and Innovation in the Republic of Ireland. Dr. Chung Loong Yiin would also like to acknowledge the technical and financial support from Universiti Malaysia Sarawak (UNIMAS).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Osman, A. ., Lai, Z.Y., Farghali, M. et al. Optimizing biomass pathways to bioenergy and biochar application in electricity generation, biodiesel production, and biohydrogen production. Environ Chem Lett 21, 2639–2705 (2023). https://doi.org/10.1007/s10311-023-01613-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-023-01613-2