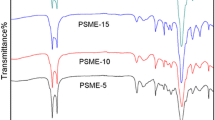
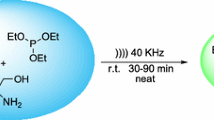
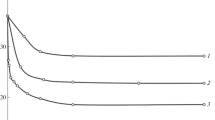
Abstract
Water is a desirable solvent for synthesis because water is cheap, safe and non-toxic. However, the use of water as solvent is limited to water-soluble compounds, thus excluding most organic, lipophilic substances. A solution to this issue is to increase the solubility of organic compounds by using a surfactant to form micelles and, in turn, increase contacts with the reagent. Here, we tested ultrasound irradiation and sodium dodecyl sulfate micellar media on Mannich reaction of aldehydes, aromatic amines and acetophenone derivatives or cyclohexanone. The sonication of biphasic systems was performed at 37 kHz, 300 Watt, under neutral conditions. Results show that the reaction is carried out readily under milder conditions. The precipitated solid Mannich product was recovered by a simple filtration without using any organic solvent. This method provides advantages such as short reaction time, mild reaction conditions and simple work-up.

Similar content being viewed by others
References
Abismail B, Canselier JP, Wilhelm AM, Delmas H, Gourdon C (1999) Emulsification by ultrasound: drop size distribution and stability. Ultrason Sonochem 6:75–83
Alegria AE, Lion Y, Kondo T, Riesz P (1989) Sonolysis of aqueous surfactant solutions: probing the interfacial region of cavitation bubbles by spin trapping. J Phys Chem 93:4908–4913
Arend M, Westermann B, Risch N (1998) Modern variants of the Mannich reaction. Angew Chem Int Ed 37:1044–1070
Cordova A (2004) The direct catalytic asymmetric Mannich reaction. Acc Chem Res 37:102–112
Firouzabadi H, Iranpoor N, Jafari AA (2005) Micellar solution of sodium dodecyl sulfate (SDS) catalyzes facile Michael addition of amines and thiols to α, β-unsaturated ketones in water under neutral conditions. Adv Synth Catal 347:655–661
Gong K, Fang D, Wang HL, Liu ZL (2007) Basic functionalized ionic liquid catalyzed one-pot Mannich-type reaction: three component synthesis of β-Amino carbonyl compounds. Monatshefte fur Chemie 138:1195–1198
Ibrahem I, Zou W, Casas J, Cordova A (2006) Direct organocatalytic enantioselective α-aminomethylation of ketones. Tetrahedron 62:357–364
Jafari AA, Mahmoudi H (2013) Room temperature aqueous Paal–Knorr pyrrole synthesis catalyzed by aluminum tris (dodecyl sulfate) trihydrate. Environ Chem Lett 11:157–162
Jafari AA, Moradgholi F (2009) Silzic (ZnCl2/SiO2) as a new and recyclable Zinc (II) catalyst for one-pot three-component syntheses of β-aminocarbonyl compounds under solvent-free conditions. Acta Chim Slov 56:744–748
Jafari AA, Moradgholi F, Tamaddon F (2009a) Pronounced catalytic effect of a micellar solution of sodium dodecylsulfate (SDS) upon a three-component reaction of aldehydes, amines, and ketones under neutral conditions. Eur J Org Chem 8:1249–1255
Jafari AA, Moradgholi F, Tamaddon F (2009b) A highly efficient Michael addition of indoles to α, β- unsaturated electron-deficient compounds in acidic SDS micellar media. J Iran Chem Soc 6:588–593
Jafari AA, Amini S, Tamaddon F (2012) A green, chemoselective and efficient protocol for Pall–Knorr pyrrole and bispyrrole synthesis using biodegradable polymeric catalyst PEG-SO3H in water. J Appl Polym Sci 125:1339–1345
Loh T, Chen S (2002) InCl3-catalyzed three-component asymmetric Mannich-type reaction in methanol. Org Lett 21:3647–3650
Manabe K, Mori Y, Kobayashi S (2001) Three-component carbon-carbon bond-forming reactions catalyzed by a brùnsted acid-surfactant-combined catalyst in water. Tetrahedron 57:2537–2544
Min S, Cui SC, Liu YH (2005) Mannich-type reaction of (1-methoxymethylpropenyloxy) trimethylsilane with arylaldehydes and aromatic amines catalyzed by perfluorinated rare earth metal salts in fluorous phase. Tetrahedron 61:4965–4970
Acknowledgments
Financial support from the Research Council of Yazd University is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghadami, M., Jafari, A.A. Efficient synthesis of Mannich bases by sonication in sodium dodecyl sulfate micellar media. Environ Chem Lett 13, 191–196 (2015). https://doi.org/10.1007/s10311-015-0495-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-015-0495-5