Abstract
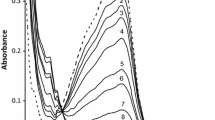
The kinetics of ferrate(VI) (FeVIO4 2−, Fe(VI)) oxidation of an antiphlogistic drug, ibuprofen (IBP), as a function of pH (7.75–9.10) and temperature (25–45°C) were investigated to see the applicability of Fe(VI) in removing this drug from water. The rates decrease with an increase in pH and the rates are related to protonation of ferrate(VI). The rates increase with an increase in temperature. The E a of the reaction at pH 9.10 was calculated as 65.4±6.4 kJ mol−1. The rate constant of the HFeO4 − with ibuprofen is lower than with the sulphur drug, sulfamethoxazole. The use of Fe(VI) to remove ibuprofen is briefly discussed.



Similar content being viewed by others
References
Caviglioli C, Valeria P, Brunella P, Sergio C, Attila A, Gaetano B (2002) Identification of degradation products of ibuprofen arising from oxidative and thermal treatments. J Pharma Biomed Anal 30:499–509
DeLuca SJ, Cantelli M, DeLuca M (1992) Ferrate vs. traditional coagulants in the treatment of combined wastes. Water Sci Technol 26:2077–2080
Doll TE, Frimmel FH (2004) Kinetic study of photocatalytic degradation of carbazepine, clofibric acid, iomerpol and iopromide assisted by different TiO2 materials—determination of intermediates and reaction pathways. Water Res 38(4):955–964
Doughton CG, Ternes TA (1999) Pharmaceuticals and personal care products in the environment: Agents of subtle change? Environ Health Perspect 107:907–908
Drewes JE, Heberer T, Rauch T, Reddersen K (2003) Fate of pharmaceuticals during ground water discharges. Ground Water Monit Remediat 23:64–72
Huber MM, Canonica S, Park G-Y, Gunten UV (2003) Oxidation of pharmaceuticals during ozonation and advanced oxidation processes. Environ Sci Technol 37:1016–1024
Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT (2002) Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: A national reconnaissance. Environ Sci Technol 36(6):1202–1211
Lopez A, Bozzi A, Mascolo G, Kiwi J (2003) Kinetics investigation on UV and UV/H2O2 degradations of pharmaceutical intermediates in aqueous solution. J Photochem Photobiol A Chem 156:121–126
Paxeus N (2000) Organic compounds in municipal landfill leachates. Water Sci Technol 42:323–333
Sharma VK, Bielski BHJ (1991) Reactivity of ferrate(VI) and ferrate(V) with amino acids. Inorg Chem 39:4306–4310
Sharma VK (2002a) Potassium ferrate(VI): An environmentally friendly oxidant. Adv Environ Res 6:143–155
Sharma VK (2002b) Ferrate(V) oxidation of pollutants: A premix pulse radiolysis. Rad Phys Chem 65:349–355
Sharma VK (2004) Use of iron(VI) and iron(V) in water and wastewater treatment. Water Sci Technol 49:69–73
Sharma VK, Mishra SK (2004) Ferrate(VI) oxidation of sulfamethoxazole: A kinetic study. In: Sharma VK, Jiang J-Q, Bouzek K (eds) Innovative ferrate(VI) technology in water and wastewater treatment, pp 102–108
Sharma VK, Kazama F, Hu J, Ray AK (2005a) Ferrates (iron(VI) and iron(V)): Environmentally-friendly oxidants and disinfectants. J Water Health 3:45–58
Sharma VK, Burnett CR, Yngard C, Cabelli D (2005b). Iron(VI) and iron(V) oxidation of copper(I) cyanide. Environ Sci Technol 39:3849–3855
Sharma VK, Mishra SK, Ray AK (2006) Kinetic assessment of the potassium ferrate(VI) oxidation of antibacterial drug sulfamethoxazole. Chemosphere 62(1):128–134
Ternes TA, Meisenheimer M, Mcdoweli D, Sacher F, Brauch H-J, Haist-Gulde B, Preuss G, Wilme U, Zulei-Seibert N (2002) Removal of pharmaceuticals during drinking water treatment. Environ Sci Technol 36:3855–3863
Thompson GW, Ockerman LT, Schreyer JM (1951) Preparation and purification of potassium ferrate VI. J Amer Chem Soc 73:1379–1381
Wood RH (1958) The heat, free energy, and entropy of the ferrate(VI) ion. J Amer Chem Soc 80:2038–2041
Zeiner C, Frimmel FH (2000) Oxidative treatment of pharmaceuticals in water. Water Res 34:1881–1885
Acknowledgment
Authors wish to thank Brandon O’Brien for useful comments on the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, V.K., Mishra, S.K. Ferrate(VI) oxidation of ibuprofen: A kinetic study. Environ Chem Lett 3, 182–185 (2006). https://doi.org/10.1007/s10311-005-0002-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-005-0002-5