Abstract
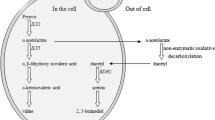
As a byproduct of yeast valine metabolism during fermentation, diacetyl can produce a buttery aroma in wine. However, high diacetyl concentrations generate an aromatic off-flavor and poor quality in wine. 2,3-Butanediol dehydrogenase encoded by BDH1 can catalyze the two reactions of acetoin from diacetyl and 2,3-butanediol from acetoin. BDH2 is a gene adjacent to BDH1, and these genes are regulated reciprocally. In this study, BDH1 and BDH2 were overexpressed in Saccharomyces uvarum to reduce the diacetyl production of wine either individually or in combination. Compared with those in the host strain WY1, the diacetyl concentrations in the recombinant strains WY1-1 with overexpressed BDH1, WY1-2 with overexpressed BDH2 alone, and WY1-12 with co-overexpressed BDH1 and BDH2 were decreased by 39.87, 33.42, and 46.71%, respectively. BDH2 was only responsible for converting diacetyl into acetoin, but not for the metabolic pathway of acetoin to 2,3-butanediol in S. uvarum. This study provided valuable insights into diacetyl reduction in wine.




Similar content being viewed by others
References
Bartowsky EJ, Costello PJ, Henschke PA (2002) Management of malolactic fermentation: wine flavour manipulation. Aust Grapegrow Winemak 461:7–14
Bartowsky EJ, Henschke PA (2000) Management of malolactic fermentation for the ‘buttery’ diacetyl flavour in wine. Aust Grapegrow Winemak 438a:58–67
Bartowsky EJ, Henschke PA (2004) The “butter” attribute of wine diacetyl desirability spoilage and beyong. Int J Food Microbiol 96(3):235–252
Blomqvist K, Suihko ML, Knowles J, Penttila M (1991) Chromosomal integration and expression of two bacterial alpha-acetolactate decarboxylase genes in brewer’s yeast. Appl Environ Microb 57(10):2796–2803
Davis CR, Wibowo D, Eschenbruch R et al (1985) Practical implications of malolactic fermentation: a review. Am J Enol Vitic 36(4):290–301
Dong J, Xu H, Zhao L et al (2014) Enhanced acetate ester production of Chinese liquor yeast by overexpressing ATF1 through precise and seamless insertion of PGK1 promoter. J Ind Microbiol Biotechnol 41(12):1823–1828
Duong CT, Strack L, Futschik M et al (2011) Identification of Sc-type ILV6 as a target to reduce diacetyl formation in lager brewers’yeast. Metab Eng 13(6):638–647
Douro A, Real V (1984) The occurrence of malolactic fermentation and diacetyl content of dry table wines from northeastern portugal. Am J Enol Vitic 35(1):49–51
Ehsani M, Fernandez M, Biosca J et al (2009) Engineering of 2,3-butanediol dehydrogenase to reduce acetoin formation by glycerol-overproducing, low-alcohol Saccharomyces cerevisiae. Appl Environ Microbiol 75(10):3196
González E, Fernández MR, Marco D et al (2010) Role of Saccharomyces cerevisiae oxidoreductases Bdh1p and Ara1p in the metabolism of acetoin and 2,3-butanediol. Appl Environ Microbiol 76(3):670
Goelling D, Stahl U (1988) Cloning and expression of an alpha-acetolactate decarboxylase gene from Streptococcus lactis subsp. diacetylactis in Escherichia coli. Appl Environ Microb 54(7):1889–1891
Goossens E, Debourg A, Villanueba K, Masschelein C (1993) Decreased diacetyl production in lager brewing yeast by integration of the ILV5 gene. Jnl Inst Brew 99(3):251–258
Guo XW, Zhang YH, Cao CH et al (2014) Enhanced production of 2,3-butanediol by overexpressing acetolactate synthase and acetoin reductase in Klebsiella pneumoniae. Biotechnol Appl Bioc 61(6):707–715
Guymon JF, Crowell EA (1965) The formation of acetoin and diacetyl during fermentation and the levels found in wines. Am J Enol Vitic 16:85–91
Haahr AM, Bredie WLP, Stahnke LH et al (2000) Flavour release of aldehydes and diacetyl in oil/water systems. Food Chem 71(3):355–362
Hayasaka Y, Bartowsky EJ (1999) Analysis of diacetyl in wine using solid-phase microextraction combined with gas chromatography–mass spectrometry. J Agric Food Chem 47(2):612–617
Kleiner D, Paul W, Merrick MJ (1988) Construction of multicopy expression vectors for regulated over-production of proteins in Klebsiella pneumoniae and other enteric bacteria. J Gen Microbiol 134(7):1779–1784
Kobayashi M, Shimizu H, Shioya S (2008) Beer volatile compounds and their application to low-malt beer fermentation. J Biosci Bioeng 106(4):317–323
Martineau B, Acree TE, Henick-Kling T (1995) Effect of wine type on the detection threshold for diacetyl. Food Res Int 28(2):139–143
Martineau B, Henick-Kling T (1995) Formation and degradation of diacetyl in wine during alcoholic fermentation with Saccharomyces cerevisiae strain EC 1118 and malolactic fermentation with Leuconostoc oenos strain MCW. Am J Enol Vitic 46:442–448
Martineau B, Henick-Kling T (2010) Performance and diacetyl production of commercial strains of malolactic bacteria in wine. J Appl Microbiol 78(5):526–536
Martineau B, Henick-Kling T, Acree T (1995) Ressessment of the influence of malolactic fermentation on the concentration of diacetyl in wines. Am J Enol Vitic 46(3):385–388
Mink R, Kölling R, Sommer S et al (2014) Diacetyl formation by Oenococcus oeni during winemaking induced by exogenous pyruvate. Am J Enol Vitic 66(1):85–90
Mink R, Sommer S, Kölling R et al (2014) Diacetyl reduction by commercial Saccharomyces cerevisiae strains during vinification. Jnl Inst Brew 120(1):23–26
Nielsen JC, Richelieu M (1999) Control of flavor development in wine during and after malolactic fermentation by Oenococcus oeni. Appl Environ Microbiol 65(2):740–745
Omura F (2008) Targeting of mitochondrial Saccharomyces cerevisiae Ilv5p to the cytosol and its effect on vicinal diketone formation in brewing. Appl Microbiol Biotechnol 78(3):503–513
Romano P, Suzzi G (1996) Origin and production of acetoin during wine yeast fermentation. Appl Environ Microbiol 62:309–315
Ramos A, Santos H (1996) Citrate and sugar cofermentation in Leuconostoc oenos, a 13C NMR study. Appl Environ Microb 62(7):2577–2585
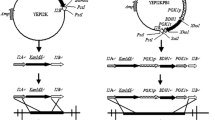
Schiestl RH, Gietz RD (1989) High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr Genet 16(5):339–346
Shi TT, Guo XW, Li P et al (2016) Diacetyl content reduction in industrial brewer’s yeast through ILV2 disruption and BDH1 expression. Eur Food Res Technol 242(6):919–926
Speckman RA, Collins EB (1968) Diacetyl biosynthesis in Streptococcus diacetilactis and Leuconostoc citrovorum. J Bacteriol 95:174–180
Teste MA, Duquenne M, François JM et al (2009) Validation of reference genes for quantitative expression analysis by real-time RT-PCR in Saccharomyces cerevisiae. BMC Mol Biol 10(1):99
Villanueba KD, Gossens E, Masschelein CA (1990) Subthreshold vicinal diketone levels in lager brewing yeast fermentations by means of ILV5 gene amplification. J Am Soc Brew Chem 48(3):111–114
Wainwright T (1973) Diacetyl—a review: part I—analytical and biochemical considerations: part II—brewing experience. J Inst Brew 79(6):451–470
Wei YJ, Hao YN, Du HJ et al (2008) Determination of diacetyl content in wine by o-phenylenediamine colorimetric method. Sino-Overseas Grapevine Wine 4:4–7 (in Chinese)
Yamaguchi M, Ishida J, Xuan ZX (1994) Determination of glyoxal, methylglyoxal, diacetyl, and 2,3-pentanedione in fermented foods by high-performance liquid chromatography with fluorescence detection. J Liq Chromatogr 17(1):203–211
Yamano S, Tanaka J, Inoue T (1994) Cloning and expression of the gene encoding alpha-acetolactate decarboxylase from Acetobacter aceti ssp. xylinum in brewer’s yeast. J Biotechnol 32(2):165–171
Yang DY, Kakuda Y, Subden RE (2006) Higher alcohols, diacetyl, acetoin and 2,3-butanediol biosynthesis in grapes undergoing carbonic maceration. Food Res Int 39(1):112–116
Zhang Y, Wang ZY, He XP, Liu N, Zhang BR (2008) New industrial brewing yeast strains with ILV2 disruption and LSD1 expression. Int J Food Microbiol 123(1–2):18–24
Zhu B, Cai G, Hall EO, Freeman GJ (2007) In-fusion™ assembly: seamless engineering of multidomain fusion proteins, modular vectors, and mutations. Biotechniques 43:354–359
Acknowledgements
The current study was financially supported by the National High Technology Research and Development Program of China (863 Program) (2012AA022108), the National Natural Science Foundation of China (31271916), and the Key Technologies R & D Program of Tianjin (Grant no. 15ZCZDNC00110).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical statement
This manuscript is in compliance with Ethical Standards. This manuscript does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, P., Guo, X., Shi, T. et al. Reducing diacetyl production of wine by overexpressing BDH1 and BDH2 in Saccharomyces uvarum . J Ind Microbiol Biotechnol 44, 1541–1550 (2017). https://doi.org/10.1007/s10295-017-1976-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-017-1976-2