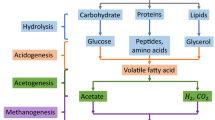
Abstract
Anaerobic digestion is a sustainable technology for the treatment of organic waste and production of biogas. Acetoclastic methanogenesis accounts for the majority of methane production in anaerobic digestion. Therefore, sustaining robust acetoclastic methanogens is important for stable process performance. Due to faster growth kinetics at high acetate concentrations, it has been considered that Methanosarcina would be more prevalent than Methanosaeta in unstable anaerobic digestion processes which frequently experience high acetate levels. Methanogen population dynamics were monitored in multiple continuous anaerobic digesters for 500 days. Results from quantitative polymerase chain reaction analysis show that Methanosaeta dominated over Methanosarcina in anaerobic digestion at high acetate levels up to 44 mM, suggesting the potential of Methanosaeta as a robust and efficient acetoclastic candidate for resilient anaerobic methane conversion. Further efforts are needed to identify mechanisms contributing to the unexpected competitiveness of these methanogens at high acetate levels observed in this study.





Similar content being viewed by others
References
Bolan N, Adriano D, Mahimairaja S (2004) Distribution and bioavailability of trace elements in livestock and poultry manure by-products. Crit Rev Environ Sci Technol 34:291–338
Chen S, Zamudio Cañas EM, Zhang Y, Zhu Z, He Q (2012) Impact of substrate overloading on archaeal populations in anaerobic digestion of animal waste. J Appl Microbiol 113:1371–1379
Chen S, Zhu Z, Park J, Zhang Z, He Q (2014) Development of Methanoculleus-specific real-time quantitative PCR assay for assessing methanogen communities in anaerobic digestion. J Appl Microbiol 116:1474–1481
Conklin A, Stensel HD, Ferguson J (2006) Growth kinetics and competition between Methanosarcina and Methanosaeta in mesophilic anaerobic digestion. Water Environ Res 78:486–496
De Vrieze J, Hennebel T, Boon N, Verstraete W (2012) Methanosarcina: the rediscovered methanogen for heavy duty biomethanation. Bioresour Technol 112:1–9
Demirel B, Scherer P (2008) The roles of acetotrophic and hydrogenotrophic methanogens during anaerobic conversion of biomass to methane: a review. Rev Environ Sci Biotechnol 7:173–190
Demirel B, Scherer P (2011) Trace element requirements of agricultural biogas digesters during biological conversion of renewable biomass to methane. Biomass Bioenerg 35:992–998
He Q, Lokken PM, Chen S, Zhou J (2009) Characterization of the impact of acetate and lactate on ethanolic fermentation by Thermoanaerobacter ethanolicus. Bioresour Technol 100:5955–5965
Kim S, Bae J, Choi O, Ju D, Lee J, Sung H, Park S, Sang B-I, Um Y (2014) A pilot scale two-stage anaerobic digester treating food waste leachate (FWL): performance and microbial structure analysis using pyrosequencing. Process Biochem 49:301–308
McHugh S, Carton M, Mahony T, O’Flaherty V (2003) Methanogenic population structure in a variety of anaerobic bioreactors. FEMS Microbiol Lett 219:297–304
McMahon KD, Zheng D, Stams AJ, Mackie RI, Raskin L (2004) Microbial population dynamics during start-up and overload conditions of anaerobic digesters treating municipal solid waste and sewage sludge. Biotechnol Bioeng 87:823–834
Moertelmaier C, Li C, Winter J, Gallert C (2014) Fatty acid metabolism and population dynamics in a wet biowaste digester during re-start after revision. Bioresour Technol 166:479–484
Moreno-Caselles J, Moral R, Perez-Murcia M, Perez-Espinosa A, Rufete B (2002) Nutrient value of animal manures in front of environmental hazards. Commun Soil Sci Plant Anal 33:3023–3032
Parkin G, Owen W (1986) Fundamentals of anaerobic digestion of wastewater sludges. J Environ Eng 112:867–920
Schnürer A, Zellner G, Svensson BH (1999) Mesophilic syntrophic acetate oxidation during methane formation in biogas reactors. FEMS Microbiol Ecol 29:249–261
Smith KS, Ingram-Smith C (2007) Methanosaeta, the forgotten methanogen? Trends Microbiol 15:150–155
Steinberg LM, Regan JM (2011) Response of lab-scale methanogenic reactors inoculated from different sources to organic loading rate shocks. Bioresour Technol 102:8790–8798
Stroot PG, McMahon KD, Mackie RI, Raskin L (2001) Anaerobic codigestion of municipal solid waste and biosolids under various mixing conditions—I. Digester performance. Water Res 35:1804–1816
Thauer RK, Kaster AK, Seedorf H, Buckel W, Hedderich R (2008) Methanogenic archaea: ecologically relevant differences in energy conservation. Nat Rev Microbiol 6:579–591
van Haandel A, De Vrieze J, Verstraete W, dos Santos VS (2013) Methanosaeta dominate acetoclastic methanogenesis during high-rate methane production in anaerobic reactors treating distillery wastewaters. J Chem Technol Biotechnol 89:1751–1759
Verstraete W, Morgan-Sagastume F, Aiyuk S, Waweru M, Rabaey K, Lissens G (2005) Anaerobic digestion as a core technology in sustainable management of organic matter. Water Sci Technol 52:59–66
Yilmaz V, Ince-Yilmaz E, Yilmazel YD, Duran M (2014) Is aceticlastic methanogen composition in full-scale anaerobic processes related to acetate utilization capacity? Appl Microbiol Biotechnol 98:5217–5226
Yu Y, Lee C, Kim J, Hwang S (2005) Group-specific primer and probe sets to detect methanogenic communities using quantitative real-time polymerase chain reaction. Biotechnol Bioeng 89:670–679
Zhang T, Fang HP (2006) Applications of real-time polymerase chain reaction for quantification of microorganisms in environmental samples. Appl Microbiol Biotechnol 70:281–289
Zhang Y, He Q (2013) Characterization of bacterial diversity in drinking water by pyrosequencing. Water Sci Technol Water Supply 13:358–367
Zhang Y, Zamudio Cañas EM, Zhu Z, Linville JL, Chen S, He Q (2011) Robustness of archaeal populations in anaerobic co-digestion of dairy and poultry wastes. Bioresour Technol 102:779–785
Zheng D, Raskin L (2000) Quantification of Methanosaeta species in anaerobic bioreactors using genus- and species-specific hybridization probes. Microb Ecol 39:246–262
Zhu Z, Hsueh MK, He Q (2011) Enhancing biomethanation of municipal waste sludge with grease trap waste as a co-substrate. Renew Energy 36:1802–1807
Acknowledgments
This work was partly supported by the Science Alliance—Tennessee Center of Excellence and the US Environmental Protection Agency Grant SU834318. SC was partly supported by the Institute for Secure and Sustainable Environment at the University of Tennessee, Knoxville.
Conflict of interest
The authors declare that they have no conflict of interest. This article does not contain any studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, S., He, Q. Persistence of Methanosaeta populations in anaerobic digestion during process instability. J Ind Microbiol Biotechnol 42, 1129–1137 (2015). https://doi.org/10.1007/s10295-015-1632-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-015-1632-7