Abstract
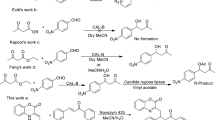
Optically pure aliphatic β-hydroxy esters were prepared from their racemates by deracemisation using the biocatalyst Candida parapsilosis ATCC 7330. High optical purity (up to >99 %) and good yields (up to 71 %) of the product secondary alcohols were obtained. This study highlights the importance of optimization of reaction conditions using ethyl-3-hydroxybutanoate as the model substrate to improve the enantioselectivity (enantiomeric excess from 9 to 98 %). The present study emphasises the broad substrate scope of the biocatalyst towards deracemisation. This is the first report of Candida parapsilosis ATCC 7330-mediated deracemisation of various alkyl-3-hydroxybutanoates to produce either the (R)-enantiomers (methyl, ethyl, propyl, butyl, t-butyl, allyl-3-hydroxybutanoates) or (S)-enantiomers (pentyl, iso-amyl and iso-propyl-3-hydroxybutanoates).





Similar content being viewed by others
References
Zhou BN, Gopalan AS, VanMiddlesworth F, Shieh WR, Sih CJ (1983) Stereochemical control of yeast reductions. 1. Asymmetric synthesis of L-carnitine. J Am Chem Soc 105(18):5925–5926
Georg GI, Kant J (1988) An asymmetric synthesis of carbapenem antibiotic (+)-PS-5 from ethyl 3-hydroxybutanoate. J Org Chem 53(3):692–695
Asako H, Shimizu M, Itoh N (2009) Biocatalytic production of (S)-4-bromo-3-hydroxybutyrate and structurally related chemicals and their applications. Appl Microbiol Biotechnol 84(3):397–405
Allegrone G, Barbeni M (1992) Identification of volatile components of caja fruit (Spondias lutea L.) and chiral analysis of 3-hydroxy aliphatic esters. Flavour Frag J 7 (6):337-342
Seebach D, Züger M (1982) Über die depolymerisierung von Poly-(R)-3-hydroxy-buttersäureester (PHB). Helv Chim Acta 65(2):495–503
Mahob RJ, Babin R, ten Hoopen GM, Dibog L, Yede, Hall DR, Bilong CF (2011) Field evaluation of synthetic sex pheromone traps for the cocoa mirid Sahlbergella singularis (Hemiptera: Miridae). Pest Manag Sci 67(6):672–676
Noyori R, Ohkuma T, Kitamura M, Takaya H, Sayo N, Kumobayashi H, Akutagawa S (1987) Asymmetric hydrogenation of β-keto carboxylic esters. A practical, purely chemical access to β-hydroxy esters in high enantiomeric purity. J Am Chem Soc 109(19):5856–5858
Corey EJ, Helal CJ (1998) Reduction of carbonyl compounds with chiral oxazaborolidine catalysts: a new paradigm for enantioselective catalysis and a powerful new synthetic method. Angew Chem Int Ed 37(15):1986–2012
Fuganti C, Grasselli P, Casati P, Carmeno M (1985) On the steric course of baker’s yeast mediated reduction of alkyl 4-azido- and 4-bromo-3-oxobutyrate. synthesis of (R)- and (S)-carnitin. Tetrahedron Lett 26(1):101–104
Nakamura K, Inoue Y, Matsuda T, Ohno A (1995) Microbial deracemization of 1-arylethanol. Tetrahedron Lett 36(35):6263–6266
Fishman A, Eroshov M, Dee-Noor SS, van Mil J, Cogan U, Effenberger R (2001) A two-step enzymatic resolution process for large-scale production of (S)- and (R)-ethyl-3-hydroxybutyrate. Biotechnol Bioeng 74(3):256–263
Strauss UT, Felfer U, Faber K (1999) Biocatalytic transformation of racemates into chiral building blocks in 100% chemical yield and 100% enantiomeric excess. Tetrahedron Asymmetry 10(1):107–117
Strauss UT, Faber K (1999) Deracemization of (±)-mandelic acid using a lipase-mandelate racemase two-enzyme system. Tetrahedron Asymmetry 10(21):4079–4081
Nie Y, Xu Y, Mu XQ (2004) Highly enantioselective conversion of racemic 1-phenyl-1,2-ethanediol by stereoinversion involving a novel cofactor-dependent oxidoreduction system of Candida parapsilosis CCTCC M203011. Org Process Res Dev 8(2):246–251
Ishige T, Honda K, Shimizu S (2005) Whole organism biocatalysis. Curr Opin Chem Biol 9:174–180
Tao J, Xu JH (2009) Biocatalysis in development of green pharmaceutical processes. Curr Opin Chem Biol 13(1):43–50
Zhang BB, Lou WY, Chen WJ, Zong MH (2012) Efficient asymmetric reduction of 4-(Trimethylsilyl)-3-butyn-2-one by Candida parapsilosis cells in an ionic liquid-containing system. PLoS One 7(5):e37641
Houng JY, Liao J-H, Wu JY, Shen SC, Hsu HF (2007) Enhancement of asymmetric bioreduction of ethyl 4-chloro acetoacetate by the design of composition of culture medium and reaction conditions. Process Biochem 42(1):1–7
Chen XH, Lou WY, Zong MH, Smith T (2011) Optimization of culture conditions to produce high yields of active Acetobacter sp. CCTCC M209061 cells for anti-Prelog reduction of prochiral ketones. BMC Biotechnol 11(1):110–121
Wang XT, Chen XH, Xu Y, Lou WY, Wu H, Zong MH (2013) Biocatalytic anti-Prelog stereoselective reduction of ethyl acetoacetate catalyzed by whole cells of Acetobacter sp. CCTCC M209061. J Biotechnol 163(3):292–300
Kaliaperumal T, Gummadi SN, Chadha A (2011) Synthesis of both enantiomers of ethyl-4-chloro-3-hydroxbutanoate from a prochiral ketone using Candida parapsilosis ATCC 7330. Tetrahedron Asymmetry 22(14–15):1548–1552
Baskar B, Pandian NG, Priya K, Chadha A (2005) Deracemisation of aryl substituted α-hydroxy esters using Candida parapsilosis ATCC 7330: effect of substrate structure and mechanism. Tetrahedron 61(52):12296–12306
Padhi SK, Chadha A (2005) Deracemisation of aromatic β-hydroxy esters using immobilised whole cells of Candida parapsilosis ATCC 7330 and determination of absolute configuration by 1H NMR. Tetrahedron Asymmetry 16(16):2790–2798
Titu D, Chadha A (2008) Enantiomerically pure allylic alcohols: preparation by Candida parapsilosis ATCC 7330 mediated deracemisation. Tetrahedron Asymmetry 19(14):1698–1701
Saravanan T, Chadha A (2010) Biocatalytic deracemization of alkyl-2-hydroxy-4-arylbut-3-ynoates using whole cells of Candida parapsilosis ATCC 7330. Tetrahedron Asymmetry 21(24):2973–2980
Kaliaperumal T, Gummadi SN, Chadha A (2011) Candida parapsilosis ATCC 7330 can also deracemise 1-arylethanols. Biocatal Biotransformation 29(6):262–270
Baskar B, Pandian NG, Priya K, Chadha A (2004) Asymmetric reduction of alkyl 2-oxo-4-arylbutanoates and -but-3-enoates by Candida parapsilosis ATCC 7330: assignment of the absolute configuration of ethyl 2-hydroxy-4-(p-methylphenyl)but-3-enoate by 1H NMR. Tetrahedron Asymmetry 15(24):3961–3966
Mahajabeen P, Chadha A (2011) One-pot synthesis of enantiomerically pure 1, 2-diols: asymmetric reduction of aromatic α-oxoaldehydes catalysed by Candida parapsilosis ATCC 7330. Tetrahedron Asymmetry 22 (24):2156–2160
Stella S, Chadha A (2012) Biocatalytic reduction of α-keto amides to (R)-α-hydroxy amides using Candida parapsilosis ATCC 7330. Catal Today 198(1):345–352
Kaliaperumal T, Kumar S, Gummadi S, Chadha A (2010) Asymmetric synthesis of (S)-ethyl-4-chloro-3-hydroxybutanoate using Candida parapsilosis ATCC 7330. J Ind Microbiol Biotechnol 37(2):159–165
Venkataraman S, Roy R, Chadha A (2013) Asymmetric reduction of alkyl-3-oxobutanoates by Candida parapsilosis ATCC 7330: insights into solvent and substrate optimisation of the biocatalytic reaction. Appl Biochem Biotechnol 171(3):756–770
Allan GR, Carnell AJ (2001) Microbial deracemization of 1-aryl and 1-heteroaryl secondary alcohols. J Org Chem 66(19):6495–6497
Hasegawa J, Ogura M, Tsuda S, Maemoto S, Kutsuki H, Ohashi T (1990) High-yield production of optically active 1,2-diols from the corresponding racemates by microbial stereoinversion. Agric Biol Chem 54(7):1819–1827
Matsuyama A, Yamamoto H, Kawada N, Kobayashi Y (2001) Industrial production of (R)-1,3-butanediol by new biocatalysts. J Mol Catal B Enzym 11(4–6):513–521
Xie SX, Ogawa J, Shimizu S (1999) Production of (R)-3-pentyn-2-ol through stereoinversion of racemic 3-pentyn-2-ol by Nocardia fusca AKU 2123. Appl Microbiol Biotechnol 52(3):327–331
Xie SX, Ogawa J, Shimizu S (1999) NAD+-dependent (S)-Specific secondary alcohol dehydrogenase involved in stereoinversion of 3-pentyn-2-ol catalyzed by Nocardia fusca AKU 2123. Biosci Biotechnol Biochem 63(10):1721–1729
Balaji BS, Chanda BM (1998) Simple and high yielding syntheses of β-keto esters catalysed by zeolites. Tetrahedron 54(43):13237–13252
Jin T, Zhang S, Li T (2002) Transesterification of β-ketoesters with alcohols catalyzed by montmorillonite K-10. Green Chem 4(1):32–34
Padhi SK, Chadha A (2003) Sodium borohydride reduction and selective transesterification of β-keto esters in a one-pot reaction under mild conditions. Synlett 05:0639–0642
Nakamura K, Fujii M, Ida Y (2001) Stereoinversion of arylethanols by Geotrichum candidum. Tetrahedron Asymmetry 12(22):3147–3153
Salvi NA, Chattopadhyay S (2004) Rhizopus arrhizus mediated asymmetric reduction of alkyl 3-oxobutanoates. Tetrahedron Asymmetry 15(21):3397–3400
Mochizuki N, Sugai T, Ohta H (1994) Biochemical reduction of 3-oxoalkanoic esters by a bottom-fermentation yeast, Saccharomyces cerevisiae IFO 0565. Biosci Biotechnol Biochem 58(9):1666–1670
Medson C, Smallridge AJ, Trewhella MA (1997) The stereoselective preparation of β-hydroxy esters using a yeast reduction in an organic solvent. Tetrahedron Asymmetry 8(7):1049–1054
Griffin DR, Gainer JL, Carta G (2001) Asymmetric ketone reduction with immobilized yeast in hexane: biocatalyst deactivation and regeneration. Biotechnol Prog 17(2):304–310
Nakamura K, Matsuda T (1998) Asymmetric reduction of ketones by the acetone powder of Geotrichum candidum. J Org Chem 63(24):8957–8964
Voss CV, Gruber CC, Kroutil W (2007) A biocatalytic one-pot oxidation/reduction sequence for the deracemisation of a sec-alcohol. Tetrahedron Asymmetry 18(2):276–281
Thangavel V, Chadha A (2007) Preparation of optically pure (3E, 5E)-alkyl-2-hydroxy-6-arylhexa-3,5-dienoates by Candida parapsilosis ATCC 7330 mediated deracemisation of the racemates. Tetrahedron 63(19):4126–4133
Keinan E, Hafeli EK, Seth KK, Lamed R (1986) Thermostable enzymes in organic synthesis. 2. Asymmetric reduction of ketones with alcohol dehydrogenase from Thermoanaerobium brockii. J Am Chem Soc 108(1):162–169
Voss CV, Gruber CC, Kroutil W (2008) Deracemization of secondary alcohols through a concurrent tandem biocatalytic oxidation and reduction. Angew Chem Intl Ed 47(4):741–745
Voss CV, Gruber CC, Faber K, Knaus T, Macheroux P, Kroutil W (2008) Orchestration of concurrent oxidation and reduction cycles for stereoinversion and deracemisation of sec-alcohols. J Am Chem Soc 130(42):13969–13972
Padhi SK, Titu D, Pandian NG, Chadha A (2006) Deracemisation of β-hydroxy esters using immobilised whole cells of Candida parapsilosis ATCC 7330: substrate specificity and mechanistic investigation. Tetrahedron 62(21):5133–5140
Acknowledgments
One of the authors (Sowmyalakshmi Venkataraman) gratefully acknowledges the Indian Institute of Technology (IIT) Madras, India for the fellowship. We thank the Sophisticated Analytical Instrumentation Facility (SAIF), IIT Madras for the NMR analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Venkataraman, S., Chadha, A. Biocatalytic deracemisation of aliphatic β-hydroxy esters: Improving the enantioselectivity by optimisation of reaction parameters. J Ind Microbiol Biotechnol 42, 173–180 (2015). https://doi.org/10.1007/s10295-014-1558-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-014-1558-5