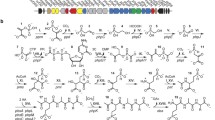
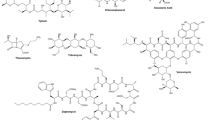
Abstract
Phosphonate natural products have proven to be a rich source of useful pharmaceutical, agricultural, and biotechnology products, whereas study of their biosynthetic pathways has revealed numerous intriguing enzymes that catalyze unprecedented biochemistry. Here we review the history of phosphonate natural product discovery, highlighting technological advances that have played a key role in the recent advances in their discovery. Central to these developments has been the application of genomics, which allowed discovery and development of a global phosphonate metabolic framework to guide research efforts. This framework suggests that the future of phosphonate natural products remains bright, with many new compounds and pathways yet to be discovered.




Similar content being viewed by others
References
Baeyer E, Gugel KH, Haegele K, Hagenmaier H, Jessipow S, Koenig WA, Zaehner J (1972) Stofwechselprodukte von Mikroorganismen 98. Phosphinothricin and phosphinothricyle-alanyl-alanin. Helv Chim Acata 55:224–239
Bérdy J (2012) Thoughts and facts about antibiotics: where we are now and where we are heading. J Antibiot 65(8):385–395
Block MD, Botterman J, Vandewiele M, Dockx J, Thoen C, Gossele V, Movva NR, Thompson C, Montagu MV, Leemans J (1987) Engineering herbicide resistance in plants by expression of a detoxifying enzyme. EMBO J 6(9):2513–2518
Blodgett JA, Zhang JK, Metcalf WW (2005) Molecular cloning, sequence analysis, and heterologous expression of the phosphinothricin tripeptide biosynthetic gene cluster from Streptomyces viridochromogenes DSM 40736. Antimicrob Agents Chemother 49(1):230–240
Blodgett JAV, Thomas PM, Li G, Velasquez JE, van der Donk WA, Kelleher NL, Metcalf WW (2007) Unusual transformations in the biosynthesis of the antibiotic phosphinothricin tripeptide. Nat Chem Biol 3:480–485 (d8f2bb67-9a19-5a0a-b099-335bae156967)
Borisova SA, Circello BT, Zhang JK, van der Donk WA, Metcalf WW (2010) Biosynthesis of rhizocticins, antifungal phosphonate oligopeptides produced by Bacillus subtilis ATCC6633. Chem Biol 17(1):28–37
Bowman E, McQueney M, Barry RJ, Dunaway-Mariano D (1988) Catalysis and thermodynamics of the phosphoenolpyruvate/phosphonopyruvate rearrangement. Entry into the phosphonate class of naturally occurring organophosphorus compounds. J Am Chem Soc 110:5575–5576
Christensen BG, Leanza WJ, Beattie TR, Patchett AA, Arison BH, Ormond RE, Kuehl FA Jr, Albers-Schonberg G, Jardetzky O (1969) Phosphonomycin: structure and synthesis. Science 166(3901):123–125
Cioni JP, Doroghazi J, Ju K-S, Yu X, Evans BS, Metcalf WW (2013) A cyanohydrin phosphonate natural product from Streptomyces regensis (under review)
Circello BT, Miller CG, Lee JH, van der Donk WA, Metcalf WW (2011) The antibiotic dehydrophos is converted to a toxic pyruvate analog by peptide bond cleavage in Salmonella enterica. Antimicrob Agents Chemother 55(7):3357–3362
D’Halluin K, De Block M, Denecke J, Janssens J, Leemans J, Reynaerts A, Botterman J (1992) The bar gene as selectable and screenable marker in plant engineering. Methods Enzymol 216:415–426
Diddens H, Zahner H, Kraas E, Gohring W, Jung G (1976) On the transport of tripeptide antibiotics in bacteria. Eur J Biochem/FEBS 66(1):11–23
Doroghazi JR, Metcalf WW (2013) Comparative genomics of actinomycetes with a focus on natural product biosynthetic genes. BMC Genomics 14(1):611
Eliot AC, Griffin BM, Thomas PM, Johannes TW, Kelleher NL, Zhao H, Metcalf WW (2008) Cloning, expression, and biochemical characterization of Streptomyces rubellomurinus genes required for biosynthesis of antimalarial compound FR900098. Chem Biol 15(8):765–770
Evans BS, Zhao C, Gao J, Evans CM, Ju KS, Doroghazi JR, van der Donk WA, Kelleher NL, Metcalf WW (2013) Discovery of the antibiotic phosacetamycin via a new mass spectrometry-based method for phosphonic acid detection. ACS Chem Biol 8(5):908–913
Fields SC (1999) Synthesis of natural products containing a C–P bond. Tetrahedron 55:12237–12273
Gao J, Ju K-S, Yu X, Velasquez JE, Mukherjee S, Lee J, Zhao C, Evans BS, Doroghazi J, Metcalf WW, van der Donk WA (2013) Use of a phosphonate methyltransferase in the identification of the fosfazinomycin biosynthetic gene cluster (under review)
Gunji S, Arima K, Beppu T (1983) Screening of antifungal antibiotics according to activities inducing morphological abnormalities. Agric Biol Chem 47:2061–2069
Hendlin D, Stapley EO, Jackson M, Wallick H, Miller AK, Wolf FJ, Miller TW, Chaiet L, Kahan FM, Foltz EL, Woodruff HB, Mata JM, Hernandez S, Mochales S (1969) Phosphonomycin, a new antibiotic produced by strains of Streptomyces. Science 166(3901):122–123
Hidaka T, Mori M, Imai S, Hara O, Nagaoka K, Seto H (1989) Studies on the biosynthesis of bialaphos (SF-1293). 9. Biochemical mechanism of C–P bond formation in bialaphos: discovery of phosphoenolpyruvate phosphomutase which catalyzes the formation of phosphonopyruvate from phosphoenolpyruvate. J Antibiot 42(3):491–494
Hilderbrand RL (1983) The role of phosphonates in living systems. CRC Press, USA
Horiguchi M, Kandatsu M (1959) Isolation of 2-aminoethane phosphonic acid from rumen protozoa. Nature 184(Suppl 12):901–902
Huerta-Cepas J, Dopazo J, Gabaldón T (2010) ETE: a python environment for tree exploration. BMC Bioinformatics 11(1):24
Hunt AH, Elzey TK (1988) Revised structure of A53868A. J Antibiot 41(6):802
Johnson R, Gordee R, Kastner R, Larsen S, Ose E (1984) Antibiotic A53868 and process for production thereof. UK Patent 2,127,413, 1984; Eli Lilly. In: Chem. Abstr, 1984. p 88837
Jomaa H, Wiesner J, Sanderbrand S, Altincicek B, Weidemeyer C, Hintz M, Turbachova I, Eberl M, Zeidler J, Lichtenthaler HK, Soldati D, Beck E (1999) Inhibitors of the nonmevalonate pathway of isoprenoid biosynthesis as antimalarial drugs. Science 285(5433):1573–1576
Katayama N, Tsubotani S, Nozaki Y, Harada S, Ono H (1990) Fosfadecin and fosfocytocin, new nucleotide antibiotics produced by bacteria. J Antibiot 43(3):238–246
Kato H, Nagayama K, Abe H, Kobayashi R, Ishihara E (1991) Isolation, structure and biological activity of trialaphos. Agric Biol Chem 55:1133–1134
Kido Y, Hamakado T, Anno M, Miyagawa E, Motoki Y, Wakamiya T, Shiba T (1984) Isolation and characterization of I5B2, a new phosphorus containing inhibitor of angiotensin I converting enzyme produced by Actinomadura sp. J Antibiot 37(9):965–969
Kim DH, Lees WJ, Kempsell KE, Lane WS, Duncan K, Walsh CT (1996) Characterization of a Cys115 to Asp substitution in the Escherichia coli cell wall biosynthetic enzyme UDP-GlcNAc enolpyruvyl transferase (MurA) that confers resistance to inactivation by the antibiotic fosfomycin. Biochemistry 35(15):4923–4928
Kim H, Chin J, Choi H, Baek K, Lee TG, Park SE, Wang W, Hahn D, Yang I, Lee J, Mun B, Ekins M, Nam SJ, Kang H (2013) Phosphoiodyns A and B, unique phosphorus-containing iodinated polyacetylenes from a Korean sponge Placospongia sp. Org Lett 15(1):100–103
Kim SY, Ju KS, Metcalf WW, Evans BS, Kuzuyama T, van der Donk WA (2012) Different biosynthetic pathways to fosfomycin in Pseudomonas syringae and Streptomyces species. Antimicrob Agents Chemother 56(8):4175–4183
Kimura T, Nakamura K, Takahashi E (1995) Phosphonothrixin, a novel herbicidal antibiotic produced by Saccharothrix sp. ST-888. II. Structure determination. J Antibiot 48(10):1130–1133
Laber B, Lindell SD, Pohlenz HD (1994) Inactivation of Escherichia coli threonine synthase by DL-Z-2-amino-5-phosphono-3-pentenoic acid. Arch Microbiol 161(5):400–403
Lee JH, Bae B, Kuemin M, Circello BT, Metcalf WW, Nair SK, van der Donk WA (2010) Characterization and structure of DhpI, a phosphonate O-methyltransferase involved in dehydrophos biosynthesis. Proc Natl Acad Sci USA 107(41):17557–17562
Lell B, Ruangweerayut R, Wiesner J, Missinou MA, Schindler A, Baranek T, Hintz M, Hutchinson D, Jomaa H, Kremsner PG (2003) Fosmidomycin, a novel chemotherapeutic agent for malaria. Antimicrob Agents Chemother 47(2):735–738
Marquardt JL, Brown ED, Lane WS, Haley TM, Ichikawa Y, Wong CH, Walsh CT (1994) Kinetics, stoichiometry, and identification of the reactive thiolate in the inactivation of UDP-GlcNAc enolpyruvoyl transferase by the antibiotic fosfomycin. Biochemistry 33(35):10646–10651
Metcalf WW, Griffin BM, Cicchillo RM, Gao J, Janga SC, Cooke HA, Circello BT, Evans BS, Martens-Habbena W, Stahl DA, van der Donk WA (2012) Synthesis of methylphosphonic acid by marine microbes: a source for methane in the aerobic ocean. Science 337(6098):1104–1107
Metcalf WW, van der Donk WA (2009) Biosynthesis of phosphonic and phosphinic acid natural products. Annu Rev Biochem 78:65–94
Moschidis MC (1985) Phosphonolipids. Prog Lipid Res 23:223–246
Ogawa H, Tsuruoka T, Inouye S, Niida T (1973) Studies on a new antibiotic SF-1293. Sci Rep Meiji Seika Kaisha 13:42–48
Ogita T, Gunji S, Fukawa Y, Terahara A, Kinoshita T, Nagaki H (1983) The structures of fosfazinomycins A and B. Tetrahedron Lett 24:2283–2286
Okuhara M, Kuroda Y, Goto T, Okamoto M, Terano H, Kohsaka M, Aoki H, Imanaka H (1980) Studies on new phosphonic acid antibiotics. I. FR-900098, isolation and characterization. J Antibiot 33(1):13–17
Okuhara M, Kuroda Y, Goto T, Okamoto M, Terano H, Kohsaka M, Aoki H, Imanaka H (1980) Studies on new phosphonic acid antibiotics. III. Isolation and characterization of FR-31564, FR-32863 and FR-33289. J Antibiot 33(1):24–28
Omura S, Hinotozawa K, Imamura N, Murata M (1984) The structure of phosalacine, a new herbicidal antibiotic containing phosphinothricin. J Antibiot 37(8):939–940
Omura S, Murata M, Hanaki H, Hinotozawa K, Oiwa R, Tanaka H (1984) Phosalacine, a new herbicidal antibiotic containing phosphinothricin. Fermentation, isolation, biological activity and mechanism of action. J Antibiot 37(8):829–835
Park BK, Hirota A, Sakai H (1976) 2-Amino-5-phosphono-3-pentenoic acid, a new amino acid from N-1409 substance, an antagonist of threonine. Agric Biol Chem 40:1905–1906
Park BK, Hirota A, Sakai H (1977) Structure of plumbemycin A and B, antagonists of l-threonine from Streptomyces plumbeus. Agric Biol Chem 41:573–579
Park BK, Hirota A, Sakai H (1977) Studies on new antimetabolite N-1409. Agric Biol Chem 41:161–167
Price MN, Dehal PS, Arkin AP (2010) FastTree 2–approximately maximum-likelihood trees for large alignments. PLoS One 5(3):e9490
Rapp C, Jung G, Kugler M, Loeffler W (1988) Rhizocticins—new phosphono-oligopeptides with antifungal activity. Liebigs Annalen der Chemie 7:655–661
Sarker SD, Latif Z, Gray AI (2005) Natural products isolation, vol 20. Springer, New York
Seidel HM, Freeman S, Seto H, Knowles JR (1988) Phosphonate biosynthesis: isolation of the enzyme responsible for the formation of a carbon–phosphorus bond. Nature 335(6189):457–458
Shigi Y (1989) Inhibition of bacterial isoprenoid synthesis by fosmidomycin, a phosphonic-acid antibiotic. J Antimicrob Chemother 24:131–145
Shoji J, Kato T, Hinoo H, Hattori T, Hirooka K, Matsumoto K, Tanimoto T, Kondo E (1986) Production of fosfomycin (phosphonomycin) by Pseudomonas syringae. J Antibiot 39(7):1011–1012
Strauch E, Wohlleben W, Puhler A (1988) Cloning of a phosphinothricin N-acetyltransferase gene from Streptomyces viridochromogenes Tu494 and its expression in Streptomyces lividans and Escherichia coli. Gene 63(1):65–74
Takahashi E, Kimura T, Nakamura K, Arahira M, Iida M (1995) Phosphonothrixin, a novel herbicidal antibiotic produced by Saccharothrix sp. ST-888. I. Taxonomy, fermentation, isolation and biological properties. J Antibiot 48(10):1124–1129
Takeuchi M, Nakajima M, Ogita T, Inukai M, Kodama K, Furuya K, Nagaki H, Haneishi T (1989) Fosfonochlorin, a new antibiotic with spheroplast forming activity. J Antibiot 42(2):198–205
Thompson CJ, Movva NR, Tizard R, Crameri R, Davies JE, Lauwereys M, Botterman J (1987) Characterization of the herbicide-resistance gene bar from Streptomyces hygroscopicus. EMBO J 6(9):2519–2523
Watanabe H, Yoshida J, Tanaka E, Ito M, Miyadoh S, Shomura T (1986) Studies on a new phosphonic acid antibiotic, SF-2312. Sci Rep Meiji Seika Kaisha 25:12–17
Widler L, Jahnke W, Green JR (2012) The chemistry of bisphosphonates: from antiscaling agents to clinical therapeutics. Anticancer Agents Med Chem 12(2):95–101
Wiesner J, Borrmann S, Jomaa H (2003) Fosmidomycin for the treatment of malaria. Parasitol Res 90(Suppl 2):S71–S76
Wohlleben W, Arnold W, Broer I, Hillemann D, Strauch E, Puhler A (1988) Nucleotide sequence of the phosphinothricin N-acetyltransferase gene from Streptomyces viridochromogenes Tu494 and its expression in Nicotiana tabacum. Gene 70(1):25–37
Woodyer RD, Shao Z, Thomas PM, Kelleher NL, Blodgett JA, Metcalf WW, van der Donk WA, Zhao H (2006) Heterologous production of fosfomycin and identification of the minimal biosynthetic gene cluster. Chem Biol 13(11):1171–1182
Yamato M, Koguchi T, Okachi R, Yamada K, Nakayama K, Kase H, Karasawa A, Shuto K (1986) K-26, a novel inhibitor of angiotensin I converting enzyme produced by an actinomycete K-26. J Antibiot 39(1):44–52
Yu X, Doroghazi JR, Janga SC, Zhang JK, Circello BT, Griffin BM, Metcalf WW (2013) Diversity and abundance of phosphonate biosynthetic genes in nature. Proc Natl Acad Sci USA (in press)
Acknowledgments
This work was supported by the National Institute of General Medical Science of the National Institutes of Health under P01GM077596 awarded to WWM. KSJ was funded by an NIH Ruth L. Kirschstein National Research Service Award (F32GM100658) from NIGMS, and JRD by an Institute for Genomic Biology Postdoctoral Fellowship. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Additional information
K.-S. Ju and J. R. Doroghazi contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ju, KS., Doroghazi, J.R. & Metcalf, W.W. Genomics-enabled discovery of phosphonate natural products and their biosynthetic pathways. J Ind Microbiol Biotechnol 41, 345–356 (2014). https://doi.org/10.1007/s10295-013-1375-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-013-1375-2