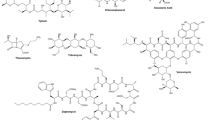
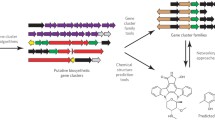
Abstract
Natural products are a large family of diverse and complex chemical molecules that have roles in both primary and secondary metabolism, and over 210,000 natural products have been described. Secondary metabolite natural products are of high commercial and societal value with therapeutic uses as antibiotics, antifungals, antitumor and antiparasitic products and in agriculture as products for crop protection and animal health. There is a resurgence of activity in exploring natural products for a wide range of applications, due to not only increasing antibiotic resistance, but the advent of next-generation genome sequencing and new technologies to interrogate and investigate natural product biosynthesis. Genome mining has revealed a previously undiscovered richness of biosynthetic potential in novel biosynthetic gene clusters for natural products. Complementing these computational processes are new experimental platforms that are being developed and deployed to access new natural products.

Similar content being viewed by others
References
Baltz RH (2017) Gifted microbes for genome mining and natural product discovery. J Ind Microbiol Biotechnol 44:573–588. https://doi.org/10.1007/s10295-016-1815-x
Baltz RH (2018) Natural product drug discovery in the genomic era: realities, conjectures, misconceptions, and opportunities. J Ind Microbiol Biotechnol. https://doi.org/10.1007/s10295-018-2115-4
Bentley SD, Chater KF, Cerdeno-Tarraga AM, Challis GL, Thomson NR, James KD, Harris DE, Quail MA, Kieser H, Harper D, Bateman A, Brown S, Chandra G, Chen CW, Collins M, Cronin A, Fraser A, Goble A, Hidalgo J, Hornsby T, Howarth S, Huang CH, Kieser T, Larke L, Murphy L, Oliver K, O’Neil S, Rabbinowitsch E, Rajandream MA, Rutherford K, Rutter S, Seeger K, Saunders D, Sharp S, Squares R, Squares S, Taylor K, Warren T, Wietzorrek A, Woodward J, Barrell BG, Parkhill J, Hopwood DA (2002) Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417:141–147. https://doi.org/10.1038/417141a
Blin K, Medema MH, Kottmann R, Lee SY, Weber T (2017) The antiSMASH database, a comprehensive database of microbial secondary metabolite biosynthetic gene clusters. Nucleic Acids Res 45:D555–d559. https://doi.org/10.1093/nar/gkw960
Blin K, Wolf T, Chevrette MG, Lu X, Schwalen CJ, Kautsar SA, Suarez Duran HG, de Los Santos ELC, Kim HU, Nave M, Dickschat JS, Mitchell DA, Shelest E, Breitling R, Takano E, Lee SY, Weber T, Medema MH (2017) AntiSMASH 4.0-improvements in chemistry prediction and gene cluster boundary identification. Nucleic Acids Res 45:W36–w41. https://doi.org/10.1093/nar/gkx319
Cohen LJ, Esterhazy D, Kim SH, Lemetre C, Aguilar RR, Gordon EA, Pickard AJ, Cross JR, Emiliano AB, Han SM, Chu J, Vila-Farres X, Kaplitt J, Rogoz A, Calle PY, Hunter C, Bitok JK, Brady SF (2017) Commensal bacteria make GPCR ligands that mimic human signalling molecules. Nature 549:48–53. https://doi.org/10.1038/nature23874
Donia MS, Fischbach MA (2015) HUMAN MICROBIOTA. Small molecules from the human microbiota. Science 349:1254766. https://doi.org/10.1126/science.1254766
Epstein SC, Charkoudian LK, Medema MH (2018) A standardized workflow for submitting data to the Minimum Information about a Biosynthetic Gene cluster (MIBiG) repository: prospects for research-based educational experiences. Stand Genom Sci 13:16. https://doi.org/10.1186/s40793-018-0318-y
Erbilgin O, Ruebel O, Louie KB, Trinh M, de Raad M, Wildish T, Udwary DW, Hoover CA, Deutsch S, Northen TR, Bowen BP (2017) MAGI: a Bayesian-like method for metabolite, annotation, and gene integration. bioRxiv. https://doi.org/10.1101/204362
Gomez-Escribano JP, Alt S, Bibb MJ (2016) Next generation sequencing of actinobacteria for the discovery of novel natural products. Mar Drugs 14:78. https://doi.org/10.3390/md14040078
Guo CJ, Chang FY, Wyche TP, Backus KM, Acker TM, Funabashi M, Taketani M, Donia MS, Nayfach S, Pollard KS, Craik CS, Cravatt BF, Clardy J, Voigt CA, Fischbach MA (2017) Discovery of reactive microbiota-derived metabolites that inhibit host proteases. Cell 168(517–526):e518. https://doi.org/10.1016/j.cell.2016.12.021
Hadjithomas M, Chen IA, Chu K, Huang J, Ratner A, Palaniappan K, Andersen E, Markowitz V, Kyrpides NC, Ivanova NN (2017) IMG–ABC: new features for bacterial secondary metabolism analysis and targeted biosynthetic gene cluster discovery in thousands of microbial genomes. Nucleic Acids Res 45:D560–d565. https://doi.org/10.1093/nar/gkw1103
Hadjithomas M, Chen IM, Chu K, Ratner A, Palaniappan K, Szeto E, Huang J, Reddy TB, Cimermancic P, Fischbach MA, Ivanova NN, Markowitz VM, Kyrpides NC, Pati A (2015) IMG–ABC: a knowledge base To fuel discovery of biosynthetic gene clusters and novel secondary metabolites. MBio 6:e00932. https://doi.org/10.1128/mBio.00932-15
Hoshino S, Onaka H, Abe I (2018) Activation of silent biosynthetic pathways and discovery of novel secondary metabolites in actinomycetes by co-culture with mycolic acid-containing bacteria. J Ind Microbiol Biotechnol. https://doi.org/10.1007/s10295-018-2100-y
Hoshino S, Wakimoto T, Onaka H, Abe I (2015) Chojalactones A–C, cytotoxic butanolides isolated from Streptomyces sp. cultivated with mycolic acid containing bacterium. Org Lett 17:1501–1504. https://doi.org/10.1021/acs.orglett.5b00385
Ikeda H, Ishikawa J, Hanamoto A, Shinose M, Kikuchi H, Shiba T, Sakaki Y, Hattori M, Omura S (2003) Complete genome sequence and comparative analysis of the industrial microorganism Streptomyces avermitilis. Nat Biotechnol 21:526–531. https://doi.org/10.1038/nbt820
Katz L, Baltz RH (2016) Natural product discovery: past, present, and future. J Ind Microbiol Biotechnol 43:155–176. https://doi.org/10.1007/s10295-015-1723-5
Keller NP (2015) Translating biosynthetic gene clusters into fungal armor and weaponry. Nat Chem Biol 11:671–677. https://doi.org/10.1038/nchembio.1897
Kim E, Moore BS, Yoon YJ (2015) Reinvigorating natural product combinatorial biosynthesis with synthetic biology. Nat Chem Biol 11:649–659. https://doi.org/10.1038/nchembio.1893
Kjærbølling I, Vesth TC, Frisvad JC, Nybo JL, Theobald S, Kuo A, Bowyer P, Matsuda Y, Mondo S, Lyhne EK, Kogle ME, Clum A, Lipzen A, Salamov A, Ngan CY, Daum C, Chiniquy J, Barry K, LaButti K, Haridas S, Simmons BA, Magnuson JK, Mortensen UH, Larsen TO, Grigoriev IV, Baker SE, Andersen MR (2018) Linking secondary metabolites to gene clusters through genome sequencing of six diverse Aspergillus species. Proc Nat Acad Sci USA. https://doi.org/10.1073/pnas.1715954115
Koh EI, Robinson AE, Bandara N, Rogers BE, Henderson JP (2017) Copper import in Escherichia coli by the yersiniabactin metallophore system. Nat Chem Biol 13:1016–1021. https://doi.org/10.1038/nchembio.2441
Li J, Wang H, Kwon YC, Jewett MC (2017) Establishing a high yielding streptomyces-based cell-free protein synthesis system. Biotechnol Bioeng 114:1343–1353. https://doi.org/10.1002/bit.26253
Li S, Li Y, Lu C, Zhang J, Zhu J, Wang H, Shen Y (2015) Activating a cryptic ansamycin biosynthetic gene cluster to produce three new naphthalenic octaketide ansamycins with n-pentyl and n-butyl side chains. Org Lett 17:3706–3709. https://doi.org/10.1021/acs.orglett.5b01686
Ling LL, Schneider T, Peoples AJ, Spoering AL, Engels I, Conlon BP, Mueller A, Schäberle TF, Hughes DE, Epstein S, Jones M, Lazarides L, Steadman VA, Cohen DR, Felix CR, Fetterman KA, Millett WP, Nitti AG, Zullo AM, Chen C, Lewis K (2015) A new antibiotic kills pathogens without detectable resistance. Nature 517:455. https://doi.org/10.1038/nature14098. https://www.nature.com/articles/nature14098#supplementary-information
Mendes R, Kruijt M, de Bruijn I, Dekkers E, van der Voort M, Schneider JH, Piceno YM, DeSantis TZ, Andersen GL, Bakker PA, Raaijmakers JM (2011) Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 332:1097–1100. https://doi.org/10.1126/science.1203980
Moore SJ, Lai HE, Needham H, Polizzi KM, Freemont PS (2017) Streptomyces venezuelae TX-TL—a next generation cell-free synthetic biology tool. Biotech J. https://doi.org/10.1002/biot.201600678
Mukherjee S, Seshadri R, Varghese NJ, Eloe-Fadrosh EA, Meier-Kolthoff JP, Goker M, Coates RC, Hadjithomas M, Pavlopoulos GA, Paez-Espino D, Yoshikuni Y, Visel A, Whitman WB, Garrity GM, Eisen JA, Hugenholtz P, Pati A, Ivanova NN, Woyke T, Klenk HP, Kyrpides NC (2017) 1,003 reference genomes of bacterial and archaeal isolates expand coverage of the tree of life. Nat Biotechnol 35:676–683. https://doi.org/10.1038/nbt.3886
Omura S, Ikeda H, Ishikawa J, Hanamoto A, Takahashi C, Shinose M, Takahashi Y, Horikawa H, Nakazawa H, Osonoe T, Kikuchi H, Shiba T, Sakaki Y, Hattori M (2001) Genome sequence of an industrial microorganism Streptomyces avermitilis: deducing the ability of producing secondary metabolites. Proc Natl Acad Sci USA 98:12215–12220. https://doi.org/10.1073/pnas.211433198
Onaka H, Mori Y, Igarashi Y, Furumai T (2011) Mycolic acid-containing bacteria induce natural-product biosynthesis in Streptomyces species. Appl Environ Microbiol 77:400–406. https://doi.org/10.1128/aem.01337-10
Papenfort K, Silpe JE, Schramma KR, Cong JP, Seyedsayamdost MR, Bassler BL (2017) A Vibrio cholerae autoinducer-receptor pair that controls biofilm formation. Nat Chem Biol 13:551–557. https://doi.org/10.1038/nchembio.2336
Patridge E, Gareiss P, Kinch MS, Hoyer D (2016) An analysis of FDA-approved drugs: natural products and their derivatives. Drug Disc Today 21:204–207. https://doi.org/10.1016/j.drudis.2015.01.009
Pishchany G, Mevers E, Ndousse-Fetter S, Horvath DJ Jr, Paludo CR, Silva-Junior EA, Koren S, Skaar EP, Clardy J, Kolter R (2018) Amycomicin is a potent and specific antibiotic discovered with a targeted interaction screen. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.1807613115
Reddy BV, Milshteyn A, Charlop-Powers Z, Brady SF (2014) eSNaPD: a versatile, web-based bioinformatics platform for surveying and mining natural product biosynthetic diversity from metagenomes. Chem Biol 21:1023–1033. https://doi.org/10.1016/j.chembiol.2014.06.007
Ren H, Wang B, Zhao H (2017) Breaking the silence: new strategies for discovering novel natural products. Curr Opin Biotechnol 48:21–27. https://doi.org/10.1016/j.copbio.2017.02.008
Rodrigues T, Reker D, Schneider P, Schneider G (2016) Counting on natural products for drug design. Nat Chem 8:531–541. https://doi.org/10.1038/nchem.2479
Shen B (2015) A new golden age of natural products drug discovery. Cell 163:1297–1300. https://doi.org/10.1016/j.cell.2015.11.031
Skinnider MA, Dejong CA, Rees PN, Johnston CW, Li H, Webster AL, Wyatt MA, Magarvey NA (2015) Genomes to natural products PRediction Informatics for Secondary Metabolomes (PRISM). Nucleic Acids Res 43:9645–9662. https://doi.org/10.1093/nar/gkv1012
Skinnider MA, Merwin NJ, Johnston CW, Magarvey NA (2017) PRISM 3: expanded prediction of natural product chemical structures from microbial genomes. Nucleic Acids Res 45:W49–w54. https://doi.org/10.1093/nar/gkx320
Smanski MJ, Zhou H, Claesen J, Shen B, Fischbach MA, Voigt CA (2016) Synthetic biology to access and expand nature’s chemical diversity. Nat Rev Microbiol 14:135–149. https://doi.org/10.1038/nrmicro.2015.24
Tietz JI, Schwalen CJ, Patel PS, Maxson T, Blair PM, Tai HC, Zakai UI, Mitchell DA (2017) A new genome-mining tool redefines the lasso peptide biosynthetic landscape. Nat Chem Biol 13:470–478. https://doi.org/10.1038/nchembio.2319
Vorholt JA (2012) Microbial life in the phyllosphere. Nat Rev Microbiol 10:828–840. https://doi.org/10.1038/nrmicro2910
Xu F, Nazari B, Moon K, Bushin LB, Seyedsayamdost MR (2017) Discovery of a cryptic antifungal compound from Streptomyces albus J1074 using high-throughput elicitor screens. J Am Chem Soc 139:9203–9212. https://doi.org/10.1021/jacs.7b02716
Zhang MM, Wong FT, Wang Y, Luo S, Lim YH, Heng E, Yeo WL, Cobb RE, Enghiad B, Ang EL, Zhao H (2017) CRISPR-Cas9 strategy for activation of silent Streptomyces biosynthetic gene clusters. Nat Chem Biol. https://doi.org/10.1038/nchembio.2341
Zhou Z, Xu Q, Bu Q, Guo Y, Liu S, Liu Y, Du Y, Li Y (2015) Genome mining-directed activation of a silent angucycline biosynthetic gene cluster in Streptomyces chattanoogensis. ChemBioChem 16:496–502. https://doi.org/10.1002/cbic.201402577
Acknowledgements
The work conducted by the U.S. Department of Energy Joint Genome Institute, a DOE Office of Science User Facility, is supported under Contract no. DE-AC02-05CH11231. We thank Richard Baltz, Leonard Katz, David Hopwood and Arnold Demain for inspiration and guidance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Special Issue “Natural Product Discovery and Development in the Genomic Era 2019”.
Rights and permissions
About this article
Cite this article
Mouncey, N.J., Otani, H., Udwary, D. et al. New voyages to explore the natural product galaxy. J Ind Microbiol Biotechnol 46, 273–279 (2019). https://doi.org/10.1007/s10295-018-02122-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-018-02122-w