Abstract
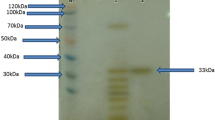
An alkaline protease from marine Engyodontium album was characterized for its physicochemical properties towards evaluation of its suitability for potential industrial applications. Molecular mass of the enzyme by matrix-assisted laser desorption ionization-mass spectrometry (MALDI-MS) analysis was calculated as 28.6 kDa. Isoelectric focusing yielded pI of 3–4. Enzyme inhibition by phenylmethylsulfonyl fluoride (PMSF) and aprotinin confirmed the serine protease nature of the enzyme. K m, V max, and K cat of the enzyme were 4.727 × 10−2 mg/ml, 394.68 U, and 4.2175 × 10−2 s−1, respectively. Enzyme was noted to be active over a broad range of pH (6–12) and temperature (15–65°C), with maximum activity at pH 11 and 60°C. CaCl2 (1 mM), starch (1%), and sucrose (1%) imparted thermal stability at 65°C. Hg2+, Cu2+, Fe3+, Zn2+, Cd+, and Al3+ inhibited enzyme activity, while 1 mM Co2+ enhanced enzyme activity. Reducing agents enhanced enzyme activity at lower concentrations. The enzyme showed considerable storage stability, and retained its activity in the presence of hydrocarbons, natural oils, surfactants, and most of the organic solvents tested. Results indicate that the marine protease holds potential for use in the detergent industry and for varied applications.






Similar content being viewed by others
References
Akasako A, Haruki M, Oobatake M, Kanaya S (1995) High-resistance of Escherichia coli ribonuclease in varient with quintuple thermostabilising mutations to thermal denaturation, acid denaturation and proteolytic degradation. Biochemistry 34:8115–8122
Ammu K, Stephen J, Devadasan K (1994) Retention of amino acids in the carcass of fish protein fed rats. Fish Technol 31:29–35
Asther H, Meunier JC (1990) Increased thermal stability of Bacillus licheniformis α-amylase in the presence of various additives. Enzyme Microb Technol 12:902–905
Barros RJ, Wehtje E, Adlercreutz P (1999) Enhancement of immobilized protease catalyzed dipeptide synthesis by the presence of insoluble protonated nucleophile. Enzyme Microb Technol 24:480–488
BCC-Business Communications Company I (2008) BIO030E-enzymes for industrial applications. Wellesley, Massachusetts
Beg QK, Gupta R (2003) Purification and characterization of an oxidation-stable, thiol-dependent serine alkaline protease from Bacillus mojavensis. Enzyme Microb Technol 32:294–304
Blumentals II, Robinson AS, Kelly RM (1990) Characterization of sodium dodecyl sulfate-resistant proteolytic activity in the hyperthermophilic archaebacterium Pyrococcus furiosus. Appl Environ Microbiol 56:1992–1998
Boskamp JV (1984) Aqueous enzyme-containing compositions with improved stability. US Patent 4,532,064
Bressollier P, Letourneau F, Urdaci M, Verneuil B (1999) Purification and characterisation of a keratinolytic serine protease from Streptomyces albidoflavus. Appl Env Microbiol 65:2570–2576
Bryan PN (2000) Protein engineering of Subtilisin. Biochem Biophys Acta Protein Struct Mol Enzyme 1543:203–222
Chellappan S, Jasmin C, Basheer SM, Elyas KK, Bhat S, Chandrasekaran M (2006) Production, purification and partial characterization of a novel protease from marine Engyodontium album BTMFS10 under solid state fermentation. Process Biochem 41:956–961
Chen ST, Wu SH, Wang KT (1991) Diastereoselective hydrolysis of peptide esters by an alkaline protease. Preparation of racemization free peptides. J Peptide Protein Res 37:347–350
Chrzanowska J, Banas J, Kolaczkowska M (2001) Purification and characterization of Beauveria bassiana proteinases. Acta Biotechnol 21:73–81
Creighton TE (1989) Protein function: a practical approach. IRL Press, Oxford
Crossin MC (1989) Protease stabilization by carboxylic-acid salts—relative efficiencies and mechanisms. J Am Oil Chem Soc 66:1010–1014
Cunningham EL, Jaswal SS, Sohl JL, Agard DA (1999) Kinetic stability as a mechanism for protease longevity. Proc Natl Acad Sci USA 96:11008–11014
Daniel RM, Cowan DA, Morgan HW, Curran MP (1982) A correlation between protein thermostability and resistance to protein lysis. Biochem J 207:641–644
Demina NS, Lysenko SV (1995) Exoproteases of Streptomyces lavendulae. Microbiology 64:385–387
Fruton S (1982) Proteinase catalysed synthesis of peptide bonds. Adv Enzymol 53:239–306
Ghorbel B, Kamoun AS, Nasri M (2003) Stability studies of protease from Bacillus cereus BG1. Enzyme Microb Technol 32:513–518
Gnosspelius G (1978) Purification and properties of an extracellular protease from Myxococcus virescens. J Bacteriol 133:826
Gold AM, Fahrney D (1964) Sulfonyl flourides as inhibitors of esterases II. Formation and reactions of phenylmethane sulfonyl alpha-chymotrypsin. Biochem 3:783–791
Gonzalez G, Gonzalez C, Merino P (1992) Thermostabilization of Cucurbita ficifolia protease in the presence of additives. Biotechnol Lett 14:919–924
Govardhan CP, Margolin AL (1995) Extremozymes for industry—from nature and by design. Chem Ind 17:689–693
Govind NS, Mehta B, Sharma M, Modi VV (1981) Protease and carotenogenesis in Blakeslea trispora. Phytochemistry 20:2483–2485
Grebeshova RN, Saldeco-Torres LE, Hidalgo MA (1999) Serine protease of Bacillus subtilis R. Appl Biochem Microbiol 35:131–134
Gupta R, Beg QK, Khan S, Chauhan B (2002) An overview on fermentation, downstream processing and properties of microbial alkaline proteases. Appl Microbiol Biotechnol 60:381–395
Gupta R, Beg QK, Lorenz P (2002) Bacterial alkaline proteases: molecular approaches and industrial applications. Appl Microbiol Biotechnol 59:15–32
Horikoshi K (1990) Enzymes of alkalophiles. Microbial Enzymes Biotechnol 2:275–294
Huang Q, Peng Y, Li X, Wang H, Zhang Y (2003) Purification and characterization of an extracellular alkaline serine protease with dehairing function from Bacillus pumilus. Curr Microbiol 46:169–173
Izotova LS, Strongin AYA, Chekulaeva LN, Sterkin VE, Ostoslavskaya VI, Timokhina EA, Stepanov VM (1983) Purification and properties of serine protease from Halobacterium halobium. J Bacteriol 155:826
James PDA, Iqbal M, Edwards C, Miller PGG (1991) Extracellular protease activity in antibiotic-producing Streptomyces thermoviolaceus. Curr Microbiol 22:377–382
Jasmin C, Chellappan S, Sukumaran RK, Elyas KK, Bhat SG, Chandrasekaran M (2010) Molecular cloning and homology modelling of a subtilisin-like serine protease from the marine fungus, Engyodontium album BTMFS10. World J Microbiol Biotechnol 26:1269–1279
Jensen ON, Shevchenko A, Mann M (1997) Protein analysis by mass spectrometry. In: Creighton TE (ed) Protein structure: a practical approach. Oxford University Press, New York
Kawashiro K, Sugahara H, Sugiyama S, Hayashi H (1997) Effect of organic solvents on enantioselectivity of protease catalysis. Biotechnol Bioeng 53:26–31
Klingeberg M, Galinsky B, Sjoholm C, Kasche V, Antranikian G (1995) Purification and properties of a highly thermostable, sodium dodecyl sulfate-resistant and stereospecific proteinase from the extremely thermophilic archaeon Thermococcus stetteri. Appl Environ Microbiol 61:3098–3104
Kobayashi T, Hakamada Y, Adachi S, Hitomi I, Yoshimatsu T, Koike K, Kawai S, Ito S (1995) Purification and properties of an alkaline protease from alkalophilic Bacillus sp. KSM-K16. Appl Microbiol Biotechnol 43:473–481
Kumar CG, Tiwari MP, Jany KD (1999) Novel alkaline serine proteases from alkalophilic Bacillus spp.: purification and some properties. Process Biochem 34:441–449
Kunitz M (1947) Crystalline soybean trypsin inhibitor II. General properties. J Gen Physiol 30:291–310
Lalonde J, Witte EJ, Oconnell ML, Holliday L (1995) Protease stabilization by highly concentrated anionic surfactant mixtures. J Am Oil Chem Soc 72:53–59
Lee KH, Lee PM, Siaw YS, Morihara K (1993) Kinetics of aspartame precursor synthesis catalysed by Pseudomonas aeruginosa elastase. J Chem Technol Biotechnol 56:375–381
Lowry OH, Rosenbrough NJ, Farr AL, Randal RJ (1951) Protein measurement with Folin phenol reagent. J Biol Chem 193:265
O’Farrells PH (1975) High resolution two-dimensional electrophoresis of proteins. J Biol Chem 250:4007–4021
Ordonez JA, Hierro EM, Bruna JM, Hoz LDEl (1999) Changes in the components of dry-fermented sausages during ripening. Crit Rev Food Sci Nutr 39:329–367
Phadatare SU, Despande MV, Srinivasan MC (1993) High activity alkaline protease from Conidiobolus coronatus (NCL 86.8.20): Enzyme production and compatibility with commercial detergents. Enzyme Microb Technol 15:72–76
Rao MB, Thanksale AM, Ghatge MS, Deshpande VV (1998) Molecular and Biotechnological aspects of microbial proteases. Microbiol Mol Biol Rev 62:597–635
Rashbehari T, Binita S, Rintu B (2003) Purification and charaterization of a protease from solid state cultures of Aspergillus parasiticus. Process Biochem 38:1553–1558
Riva S, Chopineau J, Kieboom APG, Klibanov AM (1988) Protease-catalyzed regioselective esterification of sugars and related compounds in anhydrous dimethylformamide. J Am Chem Soc 110:584–589
Sachdev A, Krishnan S (1997) Heavy-duty liqiud detergents. Liqiud detergents. Marcel Dekker, New York, pp 261–324
Saeki K, Hitomi J, Okuda M, Hatada Y, Kageyama H, Takaiwa M, Kubota H, Hagihara H, Kobayashi T, Kawai S, Ito S (2002) A novel species of alkaliphilic Bacillus that produces an oxidatively stable alkaline serine protease. Extremophiles 6:65–72
Samal B, Karan B, Parker C, Stabinsky Y (1991) Isolation and thermal stability studies of two novel serine proteinases from the fungus Tritirachium album Limber. Enzyme Microb Technol 13:66–70
Severson RG (1984) Liqiud detergents containing boric acid to stabilize enzymes. US Patent 4,537,706
Sigman DS, Mooser G (1975) Chemical studies of enzyme active sites. Annu Rev Biochem 44:889–931
So JE, Shin JS, Kim BG (2000) Protease-catalyzed tripeptide (RGD) synthesis. Enzyme Microb Technol 26:108–114
Steele DB, Fiske MJ, Steele BP, Kelley VC (1992) Production of a low molecular weight, alkaline active, thermostable protease by a novel spiral-shaped bacterium, Kurthia spiroforme sp.nov. Enzyme Microb Technol 14:358–360
Timasheff SN (1998) Control of protein stability and reactions by weakly interacting cosolvents: the simplicity of the complicated. Adv Protein Chem 51:355–432
Urtz BE, Rice WC (2000) Purification and characterization of a novel extracellular protease from Beauveria bassiana. Mycol Res 104:180–186
Vallee BL, Ulmer DD (1972) Biochemical effects of mercury, cadmium and lead. Annu Rev Biochem 41:91
Wiseman A (1985) Handbook of enzyme biotechnology. Ellis Horwood, England, p 457
Wyman J, Gill SJ (1990) Binding and linkage: functional chemistry of biological macromolecules. University Science Books, Mill Valley
Acknowledgments
The authors gratefully acknowledge the financial support from Department of Biotechnology, Government of India (Sanction Order No.: BT/PR2203/AAQ/03/109/2000).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chellappan, S., Jasmin, C., Basheer, S.M. et al. Characterization of an extracellular alkaline serine protease from marine Engyodontium album BTMFS10. J Ind Microbiol Biotechnol 38, 743–752 (2011). https://doi.org/10.1007/s10295-010-0914-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-010-0914-3