Abstract
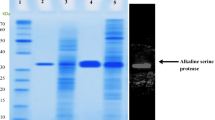
An extracellular alkaline serine protease (called DHAP), produced by a Bacillus pumilus strain, demonstrates significant dehairing function. This protease is purified by hydrophobic interaction chromatography, ion exchange, and gel filtration. DHAP had a pI of 9.0 and a molecular weight of approximately 32,000 Dalton. It shows maximal activity at pH 10 and with a temperature of 55°C; the enzyme activity can be completely inhibited by phenylmethylsulfonyl fluoride (PMSF) and diisopropyl fluorophosphates (DFP). The first 20 amino acid residues of the purified DHAP have been determined with a sequence of AQTVPYGIPQIKAPAVHAQG. Alignment of this sequence with other alkaline protease demonstrates its high homology with protease from another B. pumilus strain.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 17 April 2002 / Accepted: 24 May 2002
Rights and permissions
About this article
Cite this article
Huang, Q., Peng, Y., Li, X. et al. Purification and Characterization of an Extracellular Alkaline Serine Protease with Dehairing Function from Bacillus pumilus . Curr Microbiol 46, 0169–0173 (2003). https://doi.org/10.1007/s00284-002-3850-2
Issue Date:
DOI: https://doi.org/10.1007/s00284-002-3850-2