Abstract
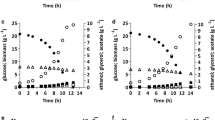
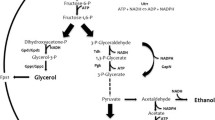
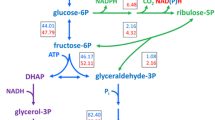
The GPD2 gene, encoding NAD+-dependent glycerol-3-phosphate dehydrogenase in an industrial ethanol-producing strain of Saccharomyces cerevisiae, was deleted. And then, either the non-phosphorylating NADP+-dependent glyceraldehyde-3-phosphate dehydrogenase (GAPN) from Bacillus cereus, or the NADP+-dependent glyceraldehyde-3-phosphate dehydrogenase (GAPDH) from Kluyveromyces lactis, was expressed in the obtained mutant AG2 deletion of GPD2, respectively. The resultant recombinant strain AG2A (gpd2Δ P PGK -gapN) exhibited a 48.70 ± 0.34% (relative to the amount of substrate consumed) decrease in glycerol production and a 7.60 ± 0.12% (relative to the amount of substrate consumed) increase in ethanol yield, while recombinant AG2B (gpd2Δ P PGK -GAPDH) exhibited a 52.90 ± 0.45% (relative to the amount of substrate consumed) decrease in glycerol production and a 7.34 ± 0.15% (relative to the amount of substrate consumed) increase in ethanol yield compared with the wild-type strain. More importantly, the maximum specific growth rates (μ max) of the recombinant AG2A and AG2B were higher than that of the mutant gpd2Δ and were indistinguishable compared with the wild-type strain in anaerobic batch fermentations. The results indicated that the redox imbalance of the mutant could be partially solved by expressing the heterologous genes.



Similar content being viewed by others
References
Albers E, Larsson C, Lidén G, Niklasson C, Gustafsson L (1996) Influence of the nitrogen source on Saccharomyces cerevisiae anaerobic growth and product formation. Appl Environ Microbiol 62:3187–3195
André L, Hemming A, Adler L (1991) Osmoregulation in Saccharomyces cerevisiae. Studies on the osmotic induction of glycerol production and glycerol 3-phosphate dehydrogenase (NAD+). FEBS Lett 286:13–17
Ansell R, Granath K, Hohmann S, Thevelein JM, Adler L (1997) The two isoenzymes for yeast NAD+-dependent glycerol 3-phosphate dehydrogenase encoded by GPD1 and GPD2 have distinct roles in osmoadaption and redox regulation. EMBO J 16:2179–2187
Arnon DI, Allen MB, Whatley FR (1954) Photosynthesis by isolated chloroplasts. Nature 173:1132–1134
Bakker BM, Overkamp KM, van Maris AJ, Kotter P, Luttik MA, van Dijken JP, Pronk JT (2001) Stoichiometry and compartmentation of NADH metabolism in Saccharomyces cerevisiae. FEMS Microbiol Rev 25:15–37
Björkqvist S, Ansell R, Adler L, Lidén G (1997) Physiological response to anaerobicity of glycerol-3-phosphate dehydrogenase mutants of Saccharomyces cerevisiae. Appl Environ Microbiol 63:128–132
Blomberg A, Adler L (1992) Physiology of osmotolerance in fungi. Adv Microb Physiol 33:146–212
Bro C, Regenberg B, Forster J, Nielsen J (2006) In silico aided metabolic engineering of Saccharomyces cerevisiae for improved bioethanol production. Metab Eng 8:102–111
Cronwright GR, Rohwer JM, Prior BA (2002) Metabolic control analysis of glycerol synthesis in Saccharomyces cerevisiae. Appl Environ Microbiol 68:4448–4456
Crow VL, Wittenberger CL (1979) Separation and properties of NAD+- and NADP+-dependent glyceraldehyde-3-phosphate dehydrogenases from Streptococcus mutans. J Biol Chem 254:1134–1142
De Vries S, van Witzenburg R, Grivell LA, Marres CAM (1992) Primary structure and import pathway of the rotenone-insensitive NADH-ubiquinone oxidoreductase of mitochondria from Saccharomyces cerevisiae. Eur J Biochem 203:587–592
Guadalupe Medina V, Almering MJ, van Maris AJ, Pronk JT (2010) Elimination of glycerol production in anaerobic cultures of a Saccharomyces cerevisiae strain engineered to use acetic acid as an electron acceptor. Appl Environ Microbiol 76:190–195
Güldener U, Heck S, Fielder T, Beinhauer J, Hegemann JH (1996) A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res 24:2519–2524
Guo ZP, Zhang L, Ding ZY, Wang ZX, Shi GY (2009) Interruption of glycerol pathway in industrial alcoholic yeasts to improve the ethanol production. Appl Microbiol Biotechnol 82:287–292
Ito H, Fukuda Y, Murata K, Kimura A (1983) Transformation of intact yeast cells treated with alkali cations. J Bacteriol 153:163–168
Iddar A, Valverde F, Serrano A, Soukri A (2002) Expression, purification, and characterization of recombinant nonphosphorylating NADP-dependent glyceraldehyde-3-phosphate dehydrogenase from Clostridium acetobutylicum. Protein Expr Purif 25:519–526
Iddar A, Valverde F, Serrano A, Soukri A (2003) Purification of recombinant non-phosphorylating NADP-dependent glyceraldehyde-3-phosphate dehydrogenase from Streptococcus pyogenes expressed in E. coli. Mol Cell Biochem 247:195–203
Larsson K, Ansell R, Eriksson P, Adler L (1993) A gene encoding sn-glycerol-3-phosphate dehydrogenase (NAD+) complements an osmosensitive mutant of Saccharomyces cerevisiae. Mol Microbiol 10:1101–1111
Nissen TL, Kielland-Brandt MC, Nielsen J, Villadsen J (2000) Optimization of ethanol production in Saccharomyces cerevisiae by metabolic engineering of the ammonium assimilation. Metab Eng 2:69–77
Nissen TL, Schulze U, Nielsen J, Villadsen J (1997) Flux distributions in anaerobic, glucose-limited continuous cultivations of Saccharomyces cerevisiae. Microbiology 143:203–218
Remize F, Barnavon L, Dequin S (2001) Glycerol export and glycerol-3-phosphate dehydrogenase, but not glycerol phosphatase, are rate limiting for glycerol production in Saccharomyces cerevisiae. Metab Eng 3:301–312
Robinson HW, Hogden CG (1940) The biuret reaction in the determination of serum proteins. I. A study of the conditions necessary for the production of a stable color which bears a quantitative relationship to the protein. J Biol Chem 135:707–1940
Valadi H, Larsson C, Gustafsson L (1998) Improved ethanol production by glycerol-3-phosphate dehydrogenase mutants of Saccharomyces cerevisiae. Appl Microbiol Biotechnol 50:434–439
Van Maris AJ, Abbott DA, Bellissimi E, Van den Brink J, Kuyper M, Luttik MA, Wisselink HW, Scheffers WA, Van Dijken JP, Pronk JT (2006) Alcoholic fermentation of carbon sources in biomass hydrolysates by Saccharomyces cerevisiae: current status. Anton Van Leeuwenhoek 90:391–418
Verduyn C, Postma E, Scheffers WA, van Dijken JP (1990) Physiology of Saccharomyces cerevisiae in anaerobic glucose-limited chemostat cultures. J Gen Microbiol 136:395–403
Verduyn C, Postma E, Scheffers WA, van Dijken JP (1992) Effect of benzoic acid on metabolic fluxes in yeasts: a continuous-culture study on the regulation of respiration and alcoholic fermentation. Yeast 8:501–517
Verduyn C, Stouthamer AH, Scheffers WA, van Dijken JP (1991) A theoretical evaluation of growth yields of yeasts. Antonie van Leeuwenhoek 59:49–63
Verho RP, Richard PH, Jonson L, Sundqvist J, Londesborough, Penttilä M (2002) Identification of the first fungal NADP-GAPDH from Kluyveromyces lactis. Biochemistry 41:13833–13838
Wang Y, Shi WL, Liu XY, Shen Y, Bao XM, Bai FW, Qu YB (2004) Establishment of a xylose metabolic pathway in an industrial strain of Saccharomyces cerevisiae. Biotechnol Lett 26:885–890
Acknowledgments
This work was financial supported by the Natural Science Foundation of China (20706024), the “863” Program (2006AA020101, 2007AA10Z359), Innovative Research Team of Jiangsu Province and China Postdoctoral Science Foundation funded project (200801361).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, Zp., Zhang, L., Ding, Zy. et al. Improving ethanol productivity by modification of glycolytic redox factor generation in glycerol-3-phosphate dehydrogenase mutants of an industrial ethanol yeast. J Ind Microbiol Biotechnol 38, 935–943 (2011). https://doi.org/10.1007/s10295-010-0864-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-010-0864-9