Abstract
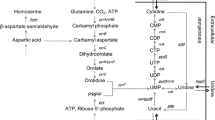
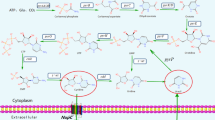
Using a simple method to introduce genetic modifications into the chromosome of naturally nontransformable Bacillus, a set of marker-free inosine-producing and 5-aminoimidazole-4-carboxamide (AICA) ribonucleoside-producing Bacillus amyloliquefaciens strains has been constructed. These strains differ in expression levels of the genes responsible for nucleoside export. Overexpression of B. amyloliquefaciens pbuE and heterologous expression of Escherichia coli nepI, which encode nucleoside efflux transporters, each notably enhanced inosine production by a B. amyloliquefaciens nucleoside-producing strain. pbuE overexpression was found to increase AICA ribonucleoside accumulation, indicating that the substrate specificity of the PbuE pump extends to this nucleoside. These results demonstrate that identifying genes whose products facilitate transport of a desired nucleoside out of cells and enhancing their expression can improve the performance of strains used for industrial production.



Similar content being viewed by others
References
Anagnostopoulos C, Spizizen J (1961) Requirements for transformation in Bacillus subtilis. J Bacteriol 81:741–746
Buhl ES, Jessen N, Pold R, Ledet T, Flyvbjerg A, Pedersen SB, Pedersen O, Schmitz O, Lund S (2002) Long-term AICAR administration reduces metabolic disturbances and lowers blood pressure in rats displaying features of the insulin resistance syndrome. Diabetes 51:2199–2206
Corton JM, Gillespie JG, Hawley SA, Hardie DG (1995) 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem 229:558–565
Diesveld R, Tietze N, Reth A, Bathe B, Sahm H, Eggeling L (2008) Activity of exporters of Escherichia coli in Corynebacterium glutamicum, and their use to increase l-threonine production. J Mol Microbiol Biotechnol 16:198–207. doi:10.1159/000142530
Doroshenko V, Airich L, Vitushkina M, Kolokolova A, Livshits V, Mashko S (2007) YddG from Escherichia coli promotes export of aromatic amino acids. FEMS Microbiol Lett 275:312–318
Galinanes M, Mullane KM, Bullough D, Hearse DJ (1992) Acadesine and myocardial protection. Studies of time of administration and dose-response relations in the rat. Circulation 86:598–608
Gronskiy SV, Zakataeva NP, Vitushkina MV, Ptitsyn LR, Altman IB, Novikova AE, Livshits VA (2005) The yicM (nepI) gene of Escherichia coli encodes a major facilitator superfamily protein involved in efflux of purine ribonucleosides. FEMS Microbiol Lett 250:39–47
Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR (1989) Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51–59
Johansen LE, Nygaard P, Lassen C, Agerso Y, Saxild HH (2003) Definition of a second Bacillus subtilis pur regulon comprising the pur and xpt-pbuX operons plus pbuG, nupG (yxjA), and pbuE (ydhL). J Bacteriol 185:5200–5209
Jomantas JAV, Fiodorova JA, Abalakina EG, Kozlov YI (1991) Genetics of Bacillus amyloliquefaciens. In: 6th international conference on Bacilli, Stanford, CA, Abstr. No. T7
Livshits VA, Zakataeva NP, Aleshin VV, Vitushkina MV (2003) Identification and characterization of the new gene rhtA involved in threonine and homoserine efflux in Escherichia coli. Res Microbiol 154:123–135
Luo Z, Saha AK, Xiang X, Ruderman NB (2005) AMPK, the metabolic syndrome and cancer. Trends Pharmacol Sci 26:69–76
Maguin E, Prevost H, Ehrlich SD, Gruss A (1996) Efficient insertional mutagenesis in lactococci and other Gram-positive bacteria. J Bacteriol 178:931–935
Mandal M, Breaker RR (2004) Adenine riboswitches and gene activation by disruption of transcription terminator. Nat Struct Biol 11:29–35
Miller JH (1972) Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, New York
Nygaard P, Saxild HH (2005) The purine efflux pump PbuE in Bacillus subtilis modulates expression of the PurR and G-box (XptR) regulons by adjusting the purine base pool size. J Bacteriol 187:791–794
Sambrook J, Russell D (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Strittmatter A, Gottschalk G, Borriss R (2007) Comparative analysis of the complete genome sequence of the plant growth promoting Bacillus amyloliquefaciens FZB42. Nat Biotechnol 25:1007–1014
Vincent MF, Marangos PJ, Gruber HE, Van den Berghe G (1991) Inhibition by AICA riboside of gluconeogenesis in isolated rat hepatocytes. Diabetes 40:1259–1266
Zakataeva NP, Aleshin VV, Tokmakova IL, Troshin PV, Livshits VA (1999) The novel transmembrane Escherichia coli proteins involved in the amino acid efflux. FEBS Lett 452:228–232
Zakataeva NP, Gronskiy SV, Sheremet AS, Kutukova EA, Novikova AE, Livshits VA (2007) A new function for the Bacillus PbuE purine base efflux pump: efflux of purine nucleosides. Res Microbiol 158:659–665
Zakataeva NP, Nikitina OV, Gronskiy SV, Romanenkov DV, Livshits VA (2010) A simple method to introduce marker-free genetic modifications into the chromosome of naturally nontransformable Bacillus amyloliquefaciens strains. Appl Microbiol Biotechnol 85:1201–1209. doi:10.1007/s00253-009-2276-1
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the BioMicroWorld 2009 Special Issue.
Rights and permissions
About this article
Cite this article
Sheremet, A.S., Gronskiy, S.V., Akhmadyshin, R.A. et al. Enhancement of extracellular purine nucleoside accumulation by Bacillus strains through genetic modifications of genes involved in nucleoside export. J Ind Microbiol Biotechnol 38, 65–70 (2011). https://doi.org/10.1007/s10295-010-0829-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-010-0829-z