Abstract
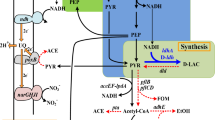
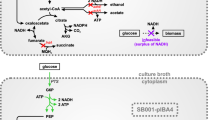
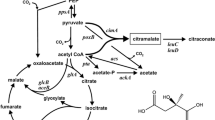
We report the conversion of glycerol to pyruvate by E. coli ALS929 containing knockouts in the genes encoding for phosphoenolpyruvate synthase, lactate dehydrogenase, pyruvate formate lyase, the pyruvate dehydrogenase complex, and pyruvate oxidase. As a result of these knockouts, ALS929 has a growth requirement of acetate for the generation of acetyl CoA. In steady-state chemostat experiments using excess glycerol and limited by acetate, lower growth rates favored the formation of pyruvate from glycerol (0.60 g/g at 0.10 h−1 versus 0.44 g/g at 0.25 h−1), while higher growth rates resulted in the maximum specific glycerol consumption rate (0.85 g/g h at 0.25 h−1 versus 0.59 g/g h at 0.10 h−1). The presence of glucose significantly improved pyruvate productivity and yield from glycerol (0.72 g/g at 0.10 h−1). In fed-batch studies using exponential acetate/glucose-limited feeding at a constant growth rate of 0.10 h−1, the final pyruvate concentration achieved was about 40 g/L in 36 h. A derivative of ALS929 which additionally knocked out methylglyoxal synthase did not further increase pyruvate productivity or yield, indicating that pyruvate formation was not limited by accumulation of methylglyoxal.


Similar content being viewed by others
References
Yazdani SS, Gonzalez R (2007) Anaerobic fermentation of glycerol: a path to economic viability for the biofuels industry. Curr Opin Biotechnol 18:213–219
Li Y, Chen J, Lun SY (2001) Biotechnological production of pyruvic acid. Appl Microbiol Biotechnol 57:451–459
Causey TB, Shanmugam KT, Yomano LP, Ingram LO (2004) Engineering Escherichia coli for efficient conversion of glucose to pyruvate. Proc Natl Acad Sci 101:2235–2240
Zelic B, Gostovic S, Vuorilehto K, Vasic-Racki B, Takors R (2004) Process strategies to enhance pyruvate production with recombinant Escherichia coli: from repetitive fed-batch to in situ product recovery with fully integrated electrodialysis. Biotechnol Bioeng 85:638–646
Yokota A, Shimizu H, Terasawa Y, Takaoka N, Tomita F (1994) Pyruvic acid production by a lipoic acid auxotroph of Escherichia coli W1485. Appl Microbiol Biotechnol 41:638–643
Yokota A, Terasawa Y, Takaoka N, Shimizu H, Tomita F (1994) Pyruvic-acid production by an F-1-atpase-defective mutant of Escherichia coli W1485lip2. Biosci Biotechnol Biochem 58:2164–2167
Tomar A, Eiteman MA, Altman E (2003) The effect of acetate pathway mutations on the production of pyruvate in Escherichia coli. Appl Microbiol Biotechnol 62:76–82
Zelic B, Gerharz T, Bott M, Vasič-Racki D, Wandrey C, Takors R (2003) Fed-batch process for pyruvate production by recombinant Escherichia coli YYC202 Strain. Eng Life Sci 3(7):299–305
Zhu Y, Eiteman MA, Altman R, Altman E (2008) High glycolytic flux improves pyruvate production by a metabolically engineered Escherichia coli strain. Appl Environ Microbiol 74:6649–6655
Saadat D, Harrison DH (1998) Identification of catalytic bases in the active site of Escherichia coli methylglyoxal synthase: cloning, expression, and functional characterization of conserved aspartic acid residues. Biochemistry 37:10074–10086
Yu D, Ellis HM, Lee E-C, Jenkins NA, Copeland NG, Court DL (2000) An efficient recombination system for chromosome engineering in Escherichia coli. Proc Natl Acad Sci USA 97(11):5978–5983
Eiteman MA, Chastain MJ (1997) Optimization of the ion-exchange analysis of organic acids from fermentation. Anal Chim Acta 338:69–75
Egli T, Lendenmann U, Snozzi M (1993) Kinetics of microbial growth with mixtures of carbon sources. Antonie Van Leeuwenhoek 63:289–298
Lendenmann U, Egli T (1995) Is Escherichai coli growing in glucose-limited chemostat culture able to utilize other sugars without lag? Microbiology 141:71–78
Underwood SA, Buszko ML, Shanmugam KT, Ingram LO (2004) Lack of protective osmolytes limits final cell density and volumetric productivity of ethanologenic Escherichia coli KO11 during xylose fermentation. Appl Environ Microbiol 70:2734–2740
Zhou S, Grabar TB, Shanmugam KT, Ingram LO (2006) Betaine tripled the volumetric productivity of d(-)-lactate by Escherichia coli strain SZ132 in mineral salts medium. Biotechnol Lett 28:671–676
MacLean MJ, Ness LS, Ferguson GP, Booth IR (1998) The role of glyoxalase I in the detoxification of methylglyoxal and in the activation of the KefB K+ efflux system in Escherichia coli. Mol Microbiol 27:563–571
Acknowledgments
The authors acknowledge the financial support of the Georgia Experiment Station. We thank Ronni Altman and Rupal Prabhu for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, Y., Eiteman, M.A., Lee, S.A. et al. Conversion of glycerol to pyruvate by Escherichia coli using acetate- and acetate/glucose-limited fed-batch processes. J Ind Microbiol Biotechnol 37, 307–312 (2010). https://doi.org/10.1007/s10295-009-0675-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-009-0675-z