Abstract
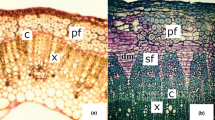
Grass lignocelluloses, such as those in corn and switchgrass, are a major resource in the emerging cellulose-to-ethanol strategy for biofuels. The potential bioconversion of carbohydrates in this potential resource, however, is limited by the associated aromatic constituents within the grass fiber. These aromatics include both lignins, which are phenylpropanoid units of various types, and low-molecular weight phenolic acids. Structural and chemical studies over the years have identified the location and limitation to fiber degradation imposed by a variety of these aromatic barriers. For example, coniferyl lignin appears to be the most effective limitation to biodegradation, existing in xylem cells of vascular tissues. On the other hand, cell walls with syringyl lignin, e.g., leaf sclerenchyma, are often less recalcitrant. Ferulic and p-coumaric acids that are esterified to hemicellulosic sugars constitute a major limitation to biodegradation in non-lignified cell walls in grass fibers, especially warm season species. Non-chemical methods to improve bioconversion of the lignocelluloses through modification of aromatics include: (1) use of lignin-degrading white rot fungi, (2) pretreatment with phenolic acid esterases, and (3) plant breeding to modify cell wall aromatics. In addition to increased availability of carbohydrates for fermentation, separation and collection of aromatics could provide value-added co-products to improve the economics of bioconversion.





Similar content being viewed by others
References
Akhtar M, Attridge MC, Myers GC, Kirk TK, Blanchette RA (1992) Biochemical pulping of loblolly pine with different strains of the white-rot fungus Ceriporiopsis subvermispora. TAPPI 75:105–109
Akin DE, Amos HE (1979) Mode of attack on orchardgrass leaf blades by rumen protozoa. Appl Environ Microbiol 37(2):332–338
Akin DE (1982) Forage cell wall degradation of orchardgrass leaf blades by rumen and sinapic acids. Agron J 74:424–428
Akin DE, Chesson A (1989) Lignification as the major factor limiting forage feeding value especially in warm condition. Proc Int Grassl Congr 16:1753–1760
Akin DE, Gordon GLR, Hogan JP (1983) Rumen bacterial and fungal degradation of Digitaria pentzii grown with or without sulfur. Appl Environ Microbiol 46(3):738–748
Akin DE, Ames-Gottfred N, Hartley RD, Fulcher RG, Rigsby LL (1990) Microspectrophotometry of phenolic compounds in bermudagrass cell walls in relation to rumen microbial digestion. Crop Sci 30:396–401
Akin DE, Hartley RD, Morrison WH III, Himmelsbach DS (1990) Diazonium compounds localize grass cell wall phenolics: relation to wall digestibility. Crop Sci 30:985–989
Akin DE, Ljungdahl LG, Wilson JR, Harris PJ (eds) (1990c). Microbial and plant opportunities to improve lignocellulose utilization by ruminants. Elsevier Science Publishing Co., New York
Akin DE, Hartley RD (1992) UV absorption microspectrophotometry and digestibility of cell types of bermudagrass internodes at different stages of maturity. J Sci Food Agric 59:437–447
Akin DE, Hartley RD (1992) Microspectrophotometry and digestibility of alkali-treated walls in bermudagrass cell types. Crop Sci 32:1116–1122
Akin DE, Rigsby LL (1992) Scanning electron microscopy and ultraviolet absorption microspectrophotometry of plant cell types of different biodegradabilities. Food Struct 11:259–271
Akin DE, Borneman WS, Rigsby LL, Martin SA (1993) p-Coumaroyl and feruloyl arabinoxylans from plant cell walls as substrates for rumen bacteria. Appl Environ Microbiol 59:644–647
Akin DE, Sethuraman A, Morrison WHIII, Martin SA, Eriksson K-EL (1993) Microbial delignification using white rot fungi improves forage digestibility. Appl Environ Microbiol 59:4274–4282
Akin DE (1995) Microspectrophotometric characterization of aromatic constituents in cell walls of hard and soft wheats. J Sci Food Agric 68:207–214
Akin DE, Morrison WH III, Rigsby LL, Gamble GR, Sethuramen A, Eriksson K-EL (1996) Biological delignification of plant components by the white rot fungi Ceriporiopsis subvermispora and Cyathus stercoreus. Anim Feed Sci Technol 63:305–321
Akin DE, Rigsby LL, Morrison WH III (2004) Oil Red as a histochemical stain for natural fibers and plant cuticle. Industr Crops Prod 19:119–124
Akin DE, Morrison WH III, Rigsby LL, Barton FE II, Himmelsbach DS, Hicks KB (2006) Corn stover fractions and bioenergy: chemical composition, structure, and response to enzyme pretreatment. Appl Biochem Biotechnol 129–132:104–116
Akin DE (2007) Grass lignocellulose: strategies to overcome recalcitrance. Appl Biochem Biotechnol 136–140:3–15
Amos HE, Akin DE (1978) Rumen protozoal degradation of structural intact forage tissues. Appl Environ Microbiol 36:513–522
Anderson WF, Peterson J, Akin DE, Morrison WH III (2005) Enzyme pretreatment of grass lignocellulose for potential high-value co-products and an improved fermentable substrate. Appl Biochem Biotechnol 121–124:303–310
Anderson WF, Dien BS, Brandon SK, Peterson JD (2007) Assessment of bermudagrass and bunch grasses as feedstock for conversion to ethanol. Appl Biochem Biotechnol. doi:10.1007/s12010-007-8041-y
Bartolomé B, Faulds CB, Tuohy M, Hazlewood GP, Gilbert HJ, Williamson G (1995) Influence of different xylanases on the activity of ferulic acid esterase on wheat bran. Biotechnol Appl Biochem 22:65–73
Beekrum S, Govinden R, Padayachee T, Odhav B (2003) Naturally occurring phenols: a detoxification strategy for fumonsin B1. Food Additives Contaminants 20:490–493
Blanchette RA, Burnes TA, Leatham GF, Effland MJ (1988) Selection of white-rot fungi for biopulping. Biomass 15:93–101
Borneman WS, Akin DE, VanEseltine WP (1986) The effect of phenolic monomers on ruminal bacteria. Appl Environ Microbiol 52:1331–1339
Borneman WS, Hartley RD, Morrison WH, Akin DE, Ljungdahl LG (1990) Feruloyl and p-coumaroyl esterase from anaerobic fungi in relation to plant cell wall degradation. Appl Environ Microbiol 33:345–351
Borneman WS, Ljungdahl LG, Hartley RD, Akin DE (1991) Isolation and Characterization of p-Coumaroyl Esterase from the Anaerobic Fungus Neocallimastix Strain MC-2. Appl Environ Microbiol 57:2337–2344
Burton GW (1972) Registration of coastcross-1 bermudagrass. Crop Sci 12:125
Burton GW, Gates RN, Hill GM (1993) Registration of Tifton 85 bermudagrass. Crop Sci 33:644–645
Carpita NC (1996) Structure and biogenesis of the cell walls of grasses. Ann Rev Plant Physiol Plant Mol Biol 47:445–476
Casler MD, Buxton DR, Vogel KP (2002) Genetic modification of lignin concentration affects fitness of perennial herbaceous plants. Theor Appl Genet 104:127–131
Casler MD, Jung HJG (2006) Relationships of fibre, lignin, and phenolics to in vitro fibre digestibility in three perennial grasses. Ani Feed Sci Technol 125:151–161
Clark G (1981) Staining Procedures. 4th edn. Biological Stain Commission, Williams and Wilkins, Baltimore
Eriksson K-EL, Blanchette RA, Ander P (1990) Microbial and enzymatic degradation of wood and wood components. Springer, New York
Faulds CB, Kroon PA, Saulnier L, Thibault J-F, Williamson G (1995) Release of ferulic acid from maize bran and derived oligosaccharides by Aspergillus niger esterases. Carbohy Polym 27:187–190
Faulds CB, Zanichelli D, Crepin VF, Connerton IF, Juge N, Bhat MK, Waldron KW (2003) Specfificity of feruloyl esterases for water-extractable and water-unextractable feruloylated polysaccharides: influence of xylanase. J Cer Sci 38:281–288
Graf E (1992) Antioxidant potential of ferulic acid. Free Radical Biol Med 13:435–448
Hanna WW, Gates RN (1990) Plant breeding to improve forage utilization. In: Akin DE, Ljungdahl LG, Wilson JR, Harris PJ (eds) Microbial and plant opportunities to improve lignocellulose utilization by ruminants. Elsevier Science Publishing Co., New York, pp 197–204
Harris PJ, Hartley RD, Barton GE (1982) Evaluation of stabilized diazoniu salts for the detection of phenolic constitutents of plant cell walls. J Sci Food Agric 33:516–520
Hartley RD, Ford CW (1989) Phenolic Constituents of plant cell walls and wal biodegradability. In: Lewis NG, Paice MG (eds) Plant cell wall polymers: biogenesis and biodegradation. American Chemical Society, Washington, DC, pp 137–145
Hartley RD, Akin DE, Himmelsbach DS, Beach DC (1990) Microspectrophotometry of bermudagrass (Cynodon dactylon) cell walls in relation to lignification and wall biodegradability. J Sci Food Agric 50:179–189
Hatfield RD, Mandebvu P, West J (1997) A comparison of Tifton 85 and Coastal bermudagrass cell walls. Annual research summary. US Dairy Forage Res Ctr Madison, WI
Hill GM, Gates RN, West JW, Watson RS, Mullinix BG (2001) Coastal and Tifton 85 hay digestion by steers: I. Cultivar and maturity effects. J Anim Sci 79:235
Jung HG, Allen MS (1995) Characteristics of plant cell walls affecting intake and digestibility of forages by ruminants. J Animal Sci 73:2774–2790
Jung H-JG, Valdez FR, Abad AR, Blanchette RA, Hatfield RD (1992) Effect of white rot basidiomycetes on chemical composition and in vitro digestibility of oat straw and alfalfa stems. J Anim Sci 70:1928–1935
Keller FA, Hamilton JE, Nguyen QA (2003) Microbial pretreatment of biomass: potential for reducing severity of thermochemical biomass pretreatment. Appl Biochem Biotech 105–108:27–41
Lazlo JA, Compton DL, Li X-L (2006) Feruloyl esterase hydrolysis and recovery of ferulic acid from jojoba meal. Ind Crops Prod 23:46–53
Mandedebvu P, West JW, Froetschel MA, Hatfield RD, Gates RN, Hill GM (1999) Effect of enzyme or microbial treatment of bermudagrass forages before ensiling on cell wall composition, end products of silage fermentation and in situ digestion kinetics. Ani Feed Sci and Tech 77:317–329
Mandedebvu P, West JW, Hill GM, Gates RN, Hatfield RD, Mullinix BG, Parks AH, Caudle AB (1999) Comparison of Tifton 85 and Coastal bermudagrasses for yield, nutrient traits, intake, and digestion by growing beef steers. J Anim Sci 77:1572–1586
Martin SA, Akin DE (1988) Effect of phenolic monomers on the growth and β-glucosidase of Bacteroides ruminicola and on the arboxymethylcellulase, β-glucosidase, and xylanase from Bacteroides succinogenes. Appl Environ Microbiol 54:3019–3022
McMillan JD (1994) Pretreatment of lignocellulosic biomass. In: Himmel ME, Baker JO, Overend RP (eds) Enzymatic conversion of biomass for fuelsproduction. American Chemical Society, Washington, DC, pp 292–324
Otjen L, Blanchette R, Effland M, Leathers G (1987) Assessment of 30 white rot basidiomycetes for selective lignin degradation. Holzforschung 41:343–349
Sarkanen KV, Ludwig CH (1971) Lignins: occurrence, formation, structure, and Reactions. Wiley, New York, pp 1–18
Stern KR, Jansky S, Bidlack JE (2003) Introductory plant biology. McGraw-Hill, New York, pp 183–184
Theodorou MK, Gascoyne DJ, Akin DE, Hartley RD (1987) Effect of phenolic acids and phenolics from plant cell walls on rumenlike fermentation in consecutive batch culture. Appl Environ Microbiol 53:1046–1050
Weymouth N, Dean JFD, Eriksson K-EL, Morrison WH III, Himmelsbach DS, Hartley RD (1993) Synthesis and spectroscopic characterization of p-hyroxyphenyl, guaiacyl and syringyl lignin polymer models (DHPs). Nordic Pulp and Paper Res J No. 4:344–349
Wilhelm WW, Johnson JMF, Hatfield JL, Voorhees WB, Linden DR (2004) Crop and soil productivity response to corn residue removal: a literature review. Agron J 96:1–17
Wubah DA, Akin DE, Borneman WS (1993) Biology, fiber-degradation, and enzymology of anaerobic zoosporic fungi. Crit Rev Microbiol 19:99–115
Vogel KP, Jung HJG (2001) Genetic modification of herbaceous plants for feed and fuel. Crit Rev Plant Sci 20:15–49
Yu P, McKinnon JJ, Maenz DD, Racz VJ, Christensen DA (2005) The specificity and the ability of Aspergillus feruloyl esterase to release p-coumaric acid from complex cell walls of oat hulls. J Chem Technol Biotechnol 79:729–733
Acknowledgments
The author of this review is indebted to many colleagues who undertook major parts of this the work or had a significant influence on the data and interpretations: (Russell Research Center) Roy D. Hartley for chemistry of cell walls, W. H. Morrison III for chemical analyses, W. S. Borneman for esterase studies, F. E. Barton II and D. S. Himmelsbach for spectroscopy and chemistry of fibers, and L. L. Rigsby for excellent technical support; (University of Georgia) K.-E. L. Eriksson for contributions on white-rot fungi, and L. G. Ljungdahl for contributions on enzymes; D. Wubah for work on anaerobic fungi; (ARS-USDA, Peoria, IL, and the University of Georgia) B. S. Dien and J. D. Peterson for ethanol conversion analyses; (ARS-USDA, Tifton, GA, USA) W. W. Hanna for work on bermudagrasses. Mention of trade names does not constitute an endorsement of one commercial product over another but is used only for identification purposes.
Author information
Authors and Affiliations
Corresponding author
Additional information
JIMB-2008: BioEnergy—Special issue.
Rights and permissions
About this article
Cite this article
Anderson, W.F., Akin, D.E. Structural and chemical properties of grass lignocelluloses related to conversion for biofuels. J Ind Microbiol Biotechnol 35, 355–366 (2008). https://doi.org/10.1007/s10295-007-0291-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-007-0291-8