Abstract
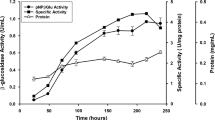
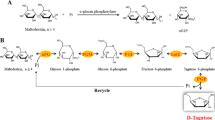
Maltodextrin phosphorylase from Pyrococcus furiosus (PF1535) was fused with the cellulose-binding domain of Clostridium cellulovorans serving as an aggregation module. After molecular cloning of the corresponding gene fusion construct and controlled expression in Escherichia coli BL21, 83% of total maltodextrin phosphorylase activity (0.24 U/mg of dry cell weight) was displayed in active inclusion bodies. These active inclusion bodies were easily isolated by nonionic detergent treatment and directly used for maltodextrin conversion to α-d-glucose-1-phosphate in a repetitive batch mode. Only 10% of enzyme activity was lost after ten conversion cycles at optimum conditions.




Similar content being viewed by others
References
Nahálka J, Shao J, Gemeiner P (2007) Oligosaccharides, neoglycoproteins and humanized plastics: their biocatalytic synthesis and possible medical applications. Biotechnol Appl Biochem 46:1–12
Kayane S, Kawai T, Sakata M, Immamura T, Tanigaki M, Kurosaki T (1996) Immobilized phosphorylase. United States Patent, US005543310A
Weinhaüsel A, Nidetzky B, Kysela C, Kulbe KD (1995) Application of Escherichia coli maltodextrin-phosphorylase for the continuous production of glucose-1-phosphate. Enzyme Microb Technol 17:140–146
Shin HJ, Shin Y, Lee DS (2000) Formation of α-d-glucose-1-phosphate by thermophilic α-1,4-d-glucan phosphorylase. J Ind Microbiol Biotechnol 24:89–93
Bae J, Lee DH, Kim D, Cho SJ, Park JE, Koh S, Kim J, Park BH, Choi Y, Shin HJ, Hong SI, Lee DS (2005) Facile synthesis of glucose-1-phosphate from starch by Thermus caldophilus GK24 α-glucan phosphorylase. Process Biochem 40:3707–3713
Chen S, Liu J, Pei H, Li J, Zhou J, Xiang H (2007) Molecular investigation of a novel thermostable glucan phosphorylase from Thermoanaerobacter tengcongensis. Enzyme Microb Technol 41:390–396
Turner P, Mamo G, Karlsson EN (2007) Potential and utilization of thermophiles and thermostable enzymes in biorefining. Microb Cell Fact 6:9
Ventura S, Villaverde A (2006) Protein quality in bacterial inclusion bodies. Trends Biotechnol 24:179–185
García-Fruitós E, González-Montalbán N, Morell M, Vera A, Ferraz RM, Arís A, Ventura S, Villaverde A (2005) Aggregation as bacterial inclusion bodies does not imply inactivation of enzymes and fluorescent proteins. Microb Cell Fact 4:27
Tokatlidis K, Dhurjati P, Millet J, Beguin P, Aubert JP (1991) High activity of inclusion bodies formed in Escherichia coli overproducing Clostridium thermocellum endoglucanase D. FEBS Lett 282:205–208
Worrall DM, Goss NH (1989) The formation of biologically active β-galactosidase inclusion bodies in Escherichia coli. Aust J Biotechnol 3:28–32
Nahálka J, Nidetzky B (2007) Fusion to a pull-down domain: a novel approach of producing Trigonopsis variabilis d-amino acid oxidase as insoluble enzyme aggregates. Biotechnol Bioeng 97:454–461
Lee HS, Shockley KR, Schut GJ, Conners SB, Montero CI, Johnson MR, Chou CJ, Bridger SL, Wigner N, Brehm SD, Jenney FE, Comfort DA, Kelly RM, Adams MWW (2006) Transcriptional and biochemical analysis of starch metabolism in the hyperthermophilic archaeon Pyrococcus furiosus. J Bacteriol 188:2115–2125
Fiala G, Stetter KO (1986) Pyrococcus furiosus sp. nov. represents a novel genus of marine heterotrophic archaebacteria growing optimally at 100°C. Arch Microbiol 145:56–61
Xavier KB, Peist R, Kossmann M, Boos W, Santos H (1999) Maltose metabolism in the hyperthermophilic archaeon Thermococcus litoralis: purification and characterization of key enzymes. J Bacteriol 181:3358–3367
Takata H, Takaha T, Okada S, Takagi M, Imanaka T (1998) Purification and characterisation of α-glucan phosphorylase from Bacillus stearothermophilus. J Ferment Bioeng 85:156–161
Bibel M, Bretl C, Gosslar U, Kriegshauser G, Liebl W (1998) Isolation and analysis of genes for amylolytic enzymes of the hyperthermophilic bacterium Thermotoga maritima. FEMS Microbiol Lett 158:9–15
Schiraldy C, Acone M (2001) Innovative fermentation strategies for the production of extremophilic enzymes. Extremophiles 5:193–198
Goedl C, Schwarz A, Minani A, Nidetzky B (2007) Recombinant sucrose phosphorylase from Leuconostoc mesenteroides: characterization, kinetic studies of transglucosylation, and application of immobilized enzyme for production of α-d-glucose-1-phosphate. J Biotechnol 129:77–86
Nahálka J, Gemeiner P (2006) Thermoswitched immobilization––a novel approach in reversible immobilization. J Biotechnol 123:478–482
Acknowledgments
The expert technical assistance of Radoslava Šályová is gratefully acknowledged. This work was supported by the Slovak Research and Development Agency under a contract no. APVV-51-040205 (http://www.biotec.chem.sk/glyctech).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nahálka, J. Physiological aggregation of maltodextrin phosphorylase from Pyrococcus furiosus and its application in a process of batch starch degradation to α-d-glucose-1-phosphate. J Ind Microbiol Biotechnol 35, 219–223 (2008). https://doi.org/10.1007/s10295-007-0287-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-007-0287-4