Abstract
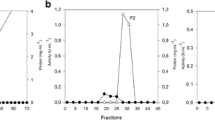
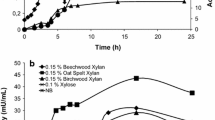
We report the purification and characterization of two thermophilic xylanases from the mesophilic bacteria Cellulomonas flavigena grown on sugarcane bagasse (SCB) as the only carbon source. Extracellular xylanase activity produced by C. flavigena was found both free in the culture supernatant and associated with residual SCB. To identify some of the molecules responsible for the xylanase activity in the substrate-bound fraction, residual SCB was treated with 3 M guanidine hydrochloride and then with 6 M urea. Further analysis of the eluted material led to the identification of two xylanases Xyl36 (36 kDa) and Xyl53 (53 kDa). The pI for Xyl36 was 5.0, while the pI for Xyl53 was 4.5. Xyl36 had a K m value of 1.95 mg/ml, while Xyl53 had a K m value of 0.78 mg/ml. In addition to SCB, Xyl36 and Xyl53 were also able to bind to insoluble oat spelt xylan and Avicel, as shown by substrate-binding assays. Xyl36 and Xyl53 showed optimal activity at pH 6.5, and at optimal temperature 65 and 55°C, respectively. Xyl36 and Xyl53 retained 24 and 35%, respectively, of their original activity after 8 h of incubation at their optimal temperature. As far as we know, this is the first study on the thermostability properties of purified xylanases from microorganisms belonging to the genus Cellulomonas.



Similar content being viewed by others
References
Amaya-Delgado L, Vega-Estrada J, Flores-Cotera LB, Dendooven L, Hidalgo-Lara ME, Montes-Horcasitas MC (2006) Induction of xylanases by sugar cane bagasse at different cell densities of Cellulomonas flavigena. Appl Microbiol Biotechnol 70(4):477–481
Beg QK, Kapoor M, Mahajan L, Hoondal GS (2001) Microbial xylanases and their industrial applications: a review. Appl Microbiol Biotechnol 56:326–338
Béguin P, Einsen H (1978) Purification and partial characterization of three extracellular cellulases from Cellulomonas sp. Eur J Biochem 87:525–531
Beily P (1993) Biochemical aspects of the production of microbial hemicellulose. In: Coughlan MP, Hazlewood GP (eds) Hemicellulose and hemicellulases. Portland Press, London, pp 29–51
Black GW, Hazlewood GP, Millward-Sadler SJ, Laurie JI, Gilbert HJ (1995) A modular xylanase containing a novel non-catalytic xylan-specific binding domain. Biochem J 307:191–195
Blanco A, Díaz P, Zueco J, Parascandola P, Pastor FIJ (1999) A multidomain xylanase from Bacillus sp with a region homologous to thermostabilizing domains of thermophilic enzymes. Microbiol 145:2163–2170
Charnock SJ, Bolam DN, Turkenburg JP, Gilbert HJ, Ferreira LMA, Davies GJ, Fontes CMGA (2000) The X6 “thermostabilizing” domains of xylanase are carbohydrate-binding modules: structure and biochemistry of the Clostridium thermocellum X6b domain. Biochem 39:5013–5021
Clark JH, Davidson K, Gilbert HJ, Fontes CMGA, Hazlewood GP (1996) A modular xylanase from mesophilic Cellulomonas fimi contains the same cellulose-binding and thermostabilizing domains as xylanases from thermophilic bacteria. FEMS Microbiol Lett 139:27–35
Dupont C, Roberge M, Shareck F, Morosoli R, Kluepfel D (1998) Substrate-binding domains of glycanases from Streptomyces lividans: characterization of a new family of xylan-binding domains. Biochem J 330:41–45
Fontes CMGA, Hazlewood GP, Morag E, Hall J, Hirst BH, Gilbert HJ (1995) Evidence for a general role for non-catalytic thermostabilizing domains in xylanases from thermophilic bacteria. J Bacteriol 307:151–158
Gupta S, Gupta MN (1993) Mechanisms of irreversible thermonoinactivation and medium engineering. In: Gupta MN (ed) Thermostability of enzymes. Narosa Publishing House, New Dehli, pp 114–122
Jørgensen H, Eriksson T, Börjensen J, Tjerneld F, Olsson L (2003) Purification and characterization of five cellulases and one xylanase from Penicillum brasilianum IBT 20888. Enzyme Microb Technol 32:851–861
Kellet LE, Poole DM, Ferreira LMA, Durrant AJ, Hazlewood GP, Gilbert HJ (1990) Xylanase B and an arabinofuranosidase from Pseudomonas fluorescens subsp. cellulose contain identical cellulose-binding domains and are encoded by adjacent genes. Biochem J 272:369–376
Khanna S, Gauri (1993) Regulation, purification and properties of xylanase from Cellulomonas fimi. Enzyme Microb Technol 15:990–995
Kolenova K, Vršanska M, Biela P (2005) Purification and characterization of two minor endo-β-1,4-xylanases of Schizophyllum commune. Enzyme Microb Technol 36:903–910
Kulkarni N, Shendye A, Rao M (2003) Molecular and biotechnological aspects of xylanases. FEMS Microbiol Rev 23:411–456
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–686
Lee SF, Forsberg CW, Rattray JB (1987) Purification and characterization of two endoxylanases from Clostridium acetobutylicum ATCC 824. Appl Environ Microbiol 53:644–650
Levy I, Shoseyov O (2002) Cellulose-binding domains: biotechnological applications. Biotechol Adv 20:191–213
Lineweaver H, Burk D (1934) The determination of enzyme dissociation constants. J Am Chem Soc 56:658–666
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Lymar ES, Li B, Renganathan V (1995) Purification and characterization of a cellulose-binding β-glucosidase from cellulose-degrading cultures of Phanerochaete chrysosporium. Appl Environ Microbiol 61:2976–2980
Martínez-Trujillo A, Pérez-Ávalos O, Ponce-Noyola T. (2003) Enzymatic properties of a purified xylanase from mutant PN-120 of Cellulomonas flavigena. Enzyme Microb Technol 32:401–406
Milagres AMF, Lacis LS, Prade RA (1993) Characterization of xylanase production by a local isolate of Penicillum janthinellum. Enzyme Microb Technol 15:248–253
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Millward-Sadler SJ, Poole DM, Henrissat B, Hazlewood GP, Clarke JH, Gilbert HJ (1994) Evidence for a general role for high-affinity non-catalytic cellulose binding domains in microbial plant cell wall hydrolases. Mol Microbiol 11:375–382
Montes HC, Ortega LJ, Magaña PI (1998) Xylanases from Cellulomonas flavigena: purification and characterization. Biotechnol Tech 12:663–666
Nakamura S, Nakai R, Wakabayashi K, Ishiguro Y, Aono R, Horikoshi K (1994) Thermophilic alkaline xylanase from newly isolated alkalophilic and thermophilic Bacillus sp. strain TAR-1. Biosci Biotechnol Biochem 58:78–81
Oku T, Roy C, Watson DC, Wakarchuk W, Campbell R, Yaguchi M, Jurasek L, Paice MG (1993) Amino acid sequence and thermostability of xylanase A from Schizophillum commune. FEBS 334:296–300
Peréz AO, Ponce NT, Magaña PI, de la Torre MM (1996) Induction of xylanase and β-xylosidase in Cellulomonas flavigena growing on different carbon sources. Appl Microbiol Biotechnol 46:405–409
Rapp P, Wagner F (1986) Production and properties of xylan-degrading enzymes from Cellulomonas uda. Appl Environ Microbiol 51:746–752
Rickard PAD, Laughlin TA (1980) Detection and assay of xylanolytic enzymes in a Cellulomona isolate. Biotechnol Lett 2:363–368
Rodríguez H, Enríquez A, Volfova O (1985) The localization and activity of Cellulomonas xylanase on sugar cane bagasse pith. Can J Microbiol 31:754–756
Sami AJ, Akhtar MW, Malik NN, Naz BA (1988) Production of free and substrate-bound cellulases of Cellulomonas flavigena. Enzyme Microb Technol 10:626–631
Sandrim VC, Rizzatti ACS, Terenzi HF, Jorge JA, Milagres AMF, Polizeli MLTM (2005) Purification and biochemical characterization of two xylanases produced by Aspergillus caespitosus and their potential for kraft pulp bleaching. Process Biochem 40:1823–1828
Schwarz WH, Bronnenmeier K, Grabnitz F, Staudenbauer WL (1987) Activity staining of cellulose in polyacrylamide gels containing mixed linkage glucans. Anal Biochem 164:72–77
Shah AR, Madamwar D (2005) Xylanase production by a newly isolated Aspergillus foetidus strain and its characterization. Process Biochem 40:1763–1771
Tan LUL, Wong KKY, Yu EKC, Saddler JN (1985) Purification and characterization of two D-xylanases from Trichoderma harzianum. Enzyme Microb Technol 7:425–430
Tan LUL, Yu EKC, Louis-Seize GW, Saddler JN (1987) Inexpensive, paid procedure for bulk purification of cellulase-free β-1,4-d-xylanase of high specific activity. Biotechnol Bioeng 30:96–100
Tomme P, Warren RAJ, Miller RC Jr, Killburn DG, Gilkes NR (1995) Cellulose-binding domains: classification and properties. In: Saddler JN, Penner MH (eds) Enzymatic degradation of insoluble carbohydrates. American Chemical Society, Washington, pp 142–163
Vega-Estrada J, Flores-Cotera LB, Santiago A, Magaña-Plaza I, Montes-Horcasitas C (2002) Draw-fill batch culture mode for production of xylanases by Cellulomonas flavigena on sugar cane bagasse. Appl Microbiol Biotechnol 58:435–438
Wong KKY, Tan LUL, Saddler JN (1988) Multiplicity of β-1,4-xylanase in microorganisms: functions and applications. Microbiol Rev 52:305–317
Yano KS, Poulos TL (2003) New understandings of thermostable, peizostable enzymes. Curr Opin Microbiol 14:360–365
Yasui T, Marui M, Kusakabe I, Nakanishi K (1988) Xylanases of Streptomyces. Methods Enzymol 160:648–654
Acknowledgments
This paper is dedicated, for the valuable teachings, to the memory of Dr. Ignacio Magaña-Plaza. We would like to thank Carmen Fontaine for the technical assistance. The research was funded by the Departamento de Biotecnología y Bioingeniería, CINVESTAV México.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Santiago-Hernández, A., Vega-Estrada, J., del Carmen Montes-Horcasitas, M. et al. Purification and characterization of two sugarcane bagasse-absorbable thermophilic xylanases from the mesophilic Cellulomonas flavigena . J Ind Microbiol Biotechnol 34, 331–338 (2007). https://doi.org/10.1007/s10295-006-0202-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-006-0202-4