Abstract
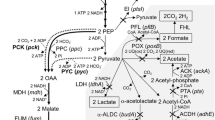
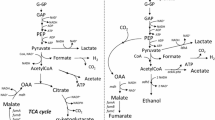
In mixed-acid fermentation, succinate synthesis requires one mole of phosphoenolpyruvate (PEP), one mole of CO2, and two moles of NADH for every mole of succinate to be formed. Different carbon sources with different properties were used to address these requirements. Sorbitol generates one more mole of NADH than glucose. Fermentation of sorbitol was shown in this study (and by others) to produce significantly more succinate than fermentation of glucose, due to increased NADH availability. Xylose fermentation conserves the intracellular PEP pool, since its transport does not require the phosphotransferase system normally used for glucose transport. The extra PEP can then be assimilated in the succinate pathway to improve production. In this study, fermentation of xylose did yield higher succinate production than glucose fermentation. Subsequent inactivation of the acetate and lactate pathways was performed to study metabolite redistribution and the effect on succinate production. With the acetate pathway inactivated, significant carbon flux shifted toward lactate rather than succinate. When both acetate and lactate pathways were inactivated, succinate yield ultimately increased with a concomitant increase in ethanol yield.

Similar content being viewed by others
References
Annette G, Pays G, Jones R, Wilkins MB, Fewson CA, Malcolm ADB (1980) Kinetic analysis of effectors of phosphoenolpyruvate carboxylase from Bryophyllum fedtschenkoi. Biochim Biophys Acta 614:151–162
Bunch PK, Mat-Jan F, Lee N, Clark DP (1997) The ldhA gene encoding the fermentative lactate dehydrogenase of Escherichia coli. Microbiology 143:187–195
Chatterjee R, Millard CS, Champion K, Clark DP, Donnelly MI (2001) Mutation of the ptsG gene results in increased production of succinate in fermentation of glucose by Escherichia coli. Appl Environ Microbiol 67:148–154
Gokarn RR, Eiteman MA, Altman E (1998) Expression of pyruvate carboxylase enhances succinate production in Escherichia coli without affecting glucose uptake. Biotechnol Lett 20:795–798
Goldberg I, Lonberg-Holm K, Bagley EA, Stieglitz B (1983) Improved conversion of fumarate to succinate by Escherichia coli strains amplified for fumarate reductase. Appl Environ Microbiol 45:1838–1847
Hong SH, Lee SY (2002) Importance of redox balance on the production of succinic acid by metabolically engineered Escherichia coli. Appl Microbiol Biotechnol 58:286–290
Li K, Frost JW (1999) Microbial synthesis of 3-dehydroshikimic acid: a comparative analysis of D-xylose, L-arabinose, and D-glucose carbon sources. Biotechnol Prog 15:876–883
Luinenberg I, Coleman JR (1992) Identification, characterization and sequence analysis of the gene encoding phosphoenolypyruvate carboxylase in Anabena sp. PCC 7120. J Gen Microbiol 138:685–691
Millard CS, Chao Y, Liao JC, Donnelly MI (1996) Enhanced production of succinic acid by overexpression of phosphoenolpyruvate carboxylase in Escherichia coli. Appl Environ Microbiol 62:1808–1810
Naide A, Izui K, Yoshinaga T (1979) Phosphoenolpyruvate carboxylase of Escherichia coli—the role of lysyl residues in the catalytic and regulatory function. J Biochem 85:423–432
Nakamura T, Yoshioka I, Takahashi M, Toh H, Izui K (1995) Cloning and sequence analysis of the gene for phosphoenolpyruvate carboxylase from an extreme thermophile, Thermus sp. J Biochem 118:319–324
Nghiem NP, Donnelly M, Millard CS, Stols L (1999) US patent 5,869,301
San K-Y, Bennett GN, Berrios-Rivera SJ, Vadali RV, Yang Y-T, Horton E, Rudolph FB, Sariyar B, Blackwood K (2002) Metabolic engineering through cofactor manipulation and its effects on metabolic flux redistribution in Escherichia coli. Metab Eng 4:182–192
Stols L, Donnelly MI (1997) Production of succinic acid through overexpression of NAD+ -dependent malic enzyme in an Escherichia coli mutant. Appl Environ Microbiol 63:2695–2701
Svensson P, Blasing OE, Westhoff P (1997) Evolution of the enzymatic characteristics of C4 phosphoenolpyruvate carboxylase—a comparison of the orthologous PPCA phosphoenolpyruvate carboxylases of Flaveria trinervia (C4) and Flaveria pringlei (C3). Eur J Biochem 246:452–460
Takai K, Sako Y, Uchida A (1998) Ppc, the gene for phosphoenolpyruvate carboxylase from an extremely thermophilic bacterium, Rhodothermus obamensis: cloning, sequence and overexpression in Escherichia coli. Microbiology 144:1423–1434
Terada K, Murata T, Izui K (1991) Site-directed mutagenesis of phosphoenolpyruvate carboxylase from E. coli: the role of His579 in the catalytic and regulatory functions. J Biochem 109:49–54
Tolentino GJ, Meng S-Y, Bennett GN, San K-Y (1992) A pH-regulated promoter for the expression of recombinant proteins in Escherichia coli. Biotechnol Lett 14:157–162
Wang X, Gong CS, Tsao GT (1998) Bioconversion of fumaric acid to succinic acid by recombinant E. coli. Appl Biochem Biotechnol 70/72:919–928
Wang Y-H, Duff SMG, Lepiniec L, Cretin C, Sarath G, Condon SA, Vidal J, Gadal P, Chollet R (1992) Site-directed mutagenesis of the phosphorylatable serine (Ser8) in C4 phosphoenolpyruvate carboxylase from Sorghum. J Biol Chem 267:16759–16762
Yang Y-T (1999) Metabolic engineering and flux analysis of Escherichia coli. PhD thesis, Rice University, Houston
Yang Y-T, San K-Y, Bennett GN (1999) Redistribution of metabolic fluxes in Escherichia coli with fermentative lactate dehydrogenase overexpression and deletion. Metab Eng 1:141–152
Zeikus JG, Jain MK, Elankovan P (1999) Biotechnology of succinic acid production and markets for derived industrial products. Appl Microbiol Biotechnol 51:545–552
Acknowledgements
The authors would like to thank Dr. Jean Vidal for providing the Sorghum pepc plasmid (pKK313). This work was supported by grants from the National Science Foundation (BES-0222691, BES-0000303). H.L. was supported by a training grant from the National Science Foundation (DGE0114264).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, H., Bennett, G.N. & San, KY. Effect of carbon sources differing in oxidation state and transport route on succinate production in metabolically engineered Escherichia coli. J IND MICROBIOL BIOTECHNOL 32, 87–93 (2005). https://doi.org/10.1007/s10295-005-0206-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-005-0206-5