Abstract
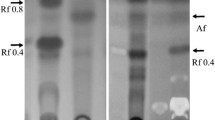
Culture supernatants of Rhodobacter sphaeroides OU5 grown in the presence of 2-aminobenzoate gave an orange-red color-reaction with Salper’s reagent, suggesting the presence of an indole derivative. This production was light-dependent and inducible only with 2-aminobenzoate. Replacement of 2-aminobenzoate with other 2-substituted benzoates did not result in the formation of indole. Fumarate appeared to be the conjugating molecule with 2-aminobenzoate, resulting in the formation of an indole derivative. The purified indole derivative was orange-brown in color, with a yields 0.34 mM from 1 mM 2-aminobenzoate. Infrared analysis suggested an indole ester and 1H NMR analysis indicated an indole carboxylate, esterified with a terpenoid alcohol. The indole ester has a mass of 441 with the molecular formula C27H39NO4. The IUPAC name of the compound is (3 E,5 E)-14-hydroxy-3,7,11-trimethyl-3,5-tetradecadienyl 2-(hydroxymethyl)-1 H-indole-3-carboxylate; and the common name given to this compound is sphestrin.





Similar content being viewed by others
References
Anderson GM (1975) Quantification of tryptophan metabolites in rat feces by thin-layer chromatography. J Chromatogr 105:323–328
Biebl H, Pfennig N (1981) Isolation of members of the family Rhodospirillaceae. In: Starr MP, Stolp H, Truper HG, Balows A, Schlegel HG (eds) The prokaryotes. Springer, Berlin Heidelberg New York, pp 167–273
Blasco R, Castillo F (1997) Characterization of 2,4-dinitrophenol uptake by Rhodobacter capsulatus. Pest Biochem Physiol 58:1–6
Chisnell JR (1984) Myo-inositol esters of indole-3-acetic acid are endogenous components of Zea mays L. shoot tissue. Plant Physiol 74:278–283
Domagalski W, Schulze A, Bandurski RS (1987) Isolation and characterization of esters of indole-3-acetic acid from the liquid endosperm of the horse chestnut (Aesculus species). Plant Physiol 84:1107–1113
Garbe TR, Kobayashi M, Shimizu, N, Takesue N, Ozawa M, Hideaki Y (2000) Indolyl carboxylic acids by condensation of indoles with α-keto acids. J Nat Prod 63:596–598
Garbe TR, Kobayashi M, Yukawa H (2000) Indole-inducible proteins in bacteria suggest membrane and oxidant toxicity. Arch Microbiol 173:78–82
Heider J, Spormann AM, Beller HR, Widdel F (1999) Anaerobic bacterial metabolism of hydrocarbons. FEMS Microbiol Rev 22:459–473
Imhoff JF (1992) Taxonomy, physiology and general ecology of anoxygenic phototrophic bacteria. In: Nicholas HM, Carr NG (eds) Photosynthetic prokaryotes. Plenum, New York, pp 53–92
Katayama M (2000) Synthesis and biological activities of 4-chloroindole-3-acetic acid and its esters. Biosci Biotechnol Biochem 64:808–815
Mollan RC, Harmey MA, Bonnelly DMX (1973) UV spectra of indoles in strong sulphuric acid. Phytochemistry 12:447–450
Nanda Devi, Sasikala Ch, Ramana Ch V (2000). Light-dependent transformation of anthranilate to indole by Rhodobacter sphaeroides OU5. J Ind Microbiol Biotechnol 24:219–221
Olgen S, Nebioglu D (2002) Synthesis and biological evaluation of N-substituted indole esters as inhibitors of cyclo-oxygenase-2 (COX-2). Farmaco 57:677–683
Rajasekhar N, Sasikala Ch, Ramana ChV (1998) Photobiotransformation of indole to its value-added derivatives by Rhodobacter sphaeroides OU5. J Ind Microbiol Biotechnol 20:177–179
Rajasekhar N, Sasikala Ch, Ramana ChV (1999) Photoproduction of indole-3-acteic acid by Rhodobacter sphaeroides from indole and glycine. Biotechnol Lett 21:543–545
Rajasekhar N, Sasikala Ch, Ramana ChV (1999) Photometabolism of indole by purple non-sulfur bacteria. Ind J Microbiol 39:39–44
Rajasekhar N, Sasikala Ch, Ramana ChV (2000) Toxicity of N-containing heterocyclic aromatic compounds and their utilization for growth by a few purple non-sulfur bacteria. Bull Environ Contam Toxicol 65:375–382
Ramana ChV, Sasikala Ch (2002) Light dependent reductive degradation of nitrobenzene by Rhodopseudomonas palustri. Ind J Exp Biol 42:229–232
Roldan MD, Blasco R, Caballero FJ, Castillo F (1998) Degradation of p-nitrophenol by the phototrophic bacterium Rhodobacter capsulatus. Arch Microbiol 169:36–42
Sasikala Ch, Ramana ChV (1998) Biodegradation and metabolism of unusual carbon compounds by anoxygenic phototrophic bacteria. Adv Microbiol Physiol 39:339–377
Wang D, Ding X, Rather PN (2001) Indole can act as an extracellular signal in Escherichia coli. J Bacteriol 183:4210–4216
Acknowledgements
M.R.S. and Ch.V.R./Ch. S. thank the Council of Scientific and Industrial Research (CSIR), Government of India, for their support through a fellowship (JRF) and research grant, respectively. Ch.V.R. and Ch.S. thank the Department of Ocean Development (DOD) and the Department of Biotechnology, Government of India, for financial support, Professor D. Basavaiah for extending facilities for 1H NMR and IR, IICT for mass analysis and the Department of Science and Technology, Government of India, for FIST support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sunayana, M.R., Sasikala, C. & Ramana, C.V. Production of a novel indole ester from 2-aminobenzoate by Rhodobacter sphaeroides OU5. J IND MICROBIOL BIOTECHNOL 32, 41–45 (2005). https://doi.org/10.1007/s10295-004-0193-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-004-0193-y