Abstract
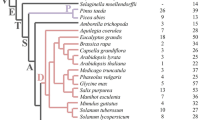
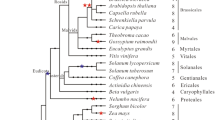
The Arabidopsis LSH1 and Oryza G1 (ALOG) protein is a family of plant-specific transcription factors that regulate reproductive growth in angiosperms. Despite their importance in plant development, little research has been conducted on ALOG proteins in basal land plants and the processes involved in their evolution remain largely unknown. Here, we studied the molecular evolution of ALOG family proteins. We found that ALOG proteins are absent in green algae but exist in all land plants analyzed as well as in some Charophycean algae, closest relatives of land plants. Multiple sequence alignments identified the high sequence conservation of ALOG domains in divergent plant lineages. Phylogenetic analyses also identified a distinct clade of ALOG protein member of lycophytes and bryophytes, including two of Marchantia polymorpha LATERAL ORGAN SUPPRESOR (MpLOS1 and MpLOS2) with a long branch length in MpLOS2. Consistent with this, the function of MpLOS1 was replaceable by Phycomitrella patens ALOG proteins, whereas MpLOS2 failed to replace the molecular function of MpLOS1. Moreover, the rice ALOG proteins, OsTAW1 and OsG1, were not able to replace the molecular function of MpLOS1 although we previously found that the function of OsG1 was replaceable by MpLOS1. Altogether, these findings suggest that ALOG proteins emerged before the evolution of land plants and that they exhibit functional conservation and diversification during the evolution of land plants. The finding that MpLOS1 is able to complement rice ALOG mutants but not vice versa also suggest the existence of conserved and the partly divergent functions of ALOG proteins in bryophytes and angiosperms.




Similar content being viewed by others
References
Bencivenga S, Serrano-Mislata A, Bush M, Fox S, Sablowski R (2016) Control of oriented tissue growth through repression of organ boundary genes promotes stem morphogenesis. Dev Cell 39:198–208
Bowman JL, Kohchi T, Yamato KT, Jenkins J, Shu S, Ishizaki K, Yamaoka S, Nishihama R, Nakamura Y, Berger F, Adam C, Aki SS, Althoff F, Araki T, Arteaga-Vazquez MA, Balasubrmanian S, Barry K, Bauer D, Boehm CR, Briginshaw L, Caballero-Perez J, Catarino B, Chen F, Chiyoda S, Chovatia M, Davies KM, Delmans M, Demura T, Dierschke T, Dolan L, Dorantes-Acosta AE, Eklund DM, Florent SN, Flores-Sandoval E, Fujiyama A, Fukuzawa H, Galik B, Grimanelli D, Grimwood J, Grossniklaus U, Hamada T, Haseloff J, Hetherington AJ, Higo A, Hirakawa Y, Hundley HN, Ikeda Y, Inoue K, Inoue SI, Ishida S, Jia Q, Kakita M, Kanazawa T, Kawai Y, Kawashima T, Kennedy M, Kinose K, Kinoshita T, Kohara Y, Koide E, Komatsu K, Kopischke S, Kubo M, Kyozuka J, Lagercrantz U, Lin SS, Lindquist E, Lipzen AM, Lu CW, De Luna E, Martienssen RA, Minamino N, Mizutani M, Mizutani M, Mochizuki N, Monte I, Mosher R, Nagasaki H, Nakagami H, Naramoto S, Nishitani K, Ohtani M, Okamoto T, Okumura M, Phillips J, Pollak B, Reinders A, Rovekamp M, Sano R, Sawa S, Schmid MW, Shirakawa M, Solano R, Spunde A, Suetsugu N, Sugano S, Sugiyama A, Sun R, Suzuki Y, Takenaka M, Takezawa D, Tomogane H, Tsuzuki M, Ueda T, Umeda M, Ward JM, Watanabe Y, Yazaki K, Yokoyama R, Yoshitake Y, Yotsui I, Zachgo S, Schmutz J (2017) Insights into land plant evolution garnered from the Marchantia polymorpha genome. Cell 171(287–304):e215
Edger PP, Pires JC (2009) Gene and genome duplications: the impact of dosage-sensitivity on the fate of nuclear genes. Chromosome Res 17:699–717
Ishizaki K, Nishihama R, Ueda M, Inoue K, Ishida S, Nishimura Y, Shikanai T, Kohchi T (2015) Development of gateway binary vector series with four different selection markers for the liverwort Marchantia polymorpha. PLoS ONE 10:e0138876
Jones VA, Dolan L (2017) MpWIP regulates air pore complex development in the liverwort Marchantia polymorpha. Development 144:1472–1476
Li X, Sun L, Tan L, Liu F, Zhu F, Zhu Z, Fu Y, Sun X, Sun X, Xie D, Sun C (2012) TH1, a DUF640 domain-like gene controls lemma and palea development in rice. Plant Mol Biol 78:351–359
MacAlister CA, Park SJ, Jiang K, Marcel F, Bendahmane A, Izkovich Y, Eshed Y, Lippman ZB (2012) Synchronization of the flowering transition by the tomato TERMINATING FLOWER gene. Nat Genet 44:1393–1398
Naramoto S, Jones VAS, Trozzi N, Sato M, Toyooka K, Shimamura M, Ishida S, Nishitani K, Ishizaki K, Nishihama R, Kohchi T, Dolan L, Kyozuka J (2019) A conserved mechanism mediates the convergent evolution of plant shoot lateral organs. PLoS Biol 17:e3000560
Peng P, Liu L, Fang J, Zhao J, Yuan S, Li X (2017) The rice TRIANGULAR HULL1 protein acts as a transcriptional repressor in regulating lateral development of spikelet. Sci Rep 7:13712
Sato D, Ohmori Y, Nagashima H, Toriba T, Hirano H (2014) A role for TRIANGULAR HULL1 in fine-tuning spikelet morphogenesis in rice. Genes Genet Syst 89:61–69
Takeda S, Hanano K, Kariya A, Shimizu S, Zhao L, Matsui M, Tasaka M, Aida M (2011) CUP-SHAPED COTYLEDON1 transcription factor activates the expression of LSH4 and LSH3, two members of the ALOG gene family, in shoot organ boundary cells. Plant J 66:1066–1077
Xu C, Park SJ, Eck JV, Lippman ZB (2018) Control of inflorescence architecture in tomato by BTB/POZ transcriptional regulators. Genes Dev 30:2048–2061
Yoshida A, Suzaki T, Tanaka W, Hirano H (2009) The homeotic gene long sterile lemma (G1) specifies sterile lemma identity in the rice spikelet. Proc Natl Acad Sci USA 106:20103–20108
Yoshida A, Sasao M, Yasuno N, Takagi K, Daimon Y, Chen R, Yamazaki R, Tokunaga H, Kitaguchi Y, Sato Y, Nagamura Y, Ushijima T, Kumamaru T, Iida S, Maekawa M, Kyozuka J (2013) TAWAWA1, a regulator of rice inflorescence architecture, functions through the suppression of meristem phase transition. Proc Natl Acad Sci USA 110:767–772
Acknowledgements
We thank Kei Saito and Eriko Kida for assistance with plant transformation. This work is supported by Grants-in-Aid from the Ministry of Education, Culture, Sports and Technology, Japan (KAKENHI Grant Numbers 17K17595 for S.N. and 17H06465 for J.K.).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Naramoto, S., Hata, Y. & Kyozuka, J. The origin and evolution of the ALOG proteins, members of a plant-specific transcription factor family, in land plants. J Plant Res 133, 323–329 (2020). https://doi.org/10.1007/s10265-020-01171-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-020-01171-6