Abstract
The effect of direct oral anticoagulants (DOACs) on turbidimetric measurements of plasma clot formation and susceptibility to fibrinolysis may facilitate a comparison between different classes of anticoagulants in plasma samples. We obtained 424 citrate plasma samples from 226 atrial fibrillation patients on anticoagulation and 24 samples without anticoagulation serving as controls. As comparators, we measured the international normalized ratio (INR) for phenprocoumon samples (N = 166), anti-Xa for low molecular weight heparin (LMWH) samples (N = 42), and DOAC levels with mass spectrometry (dabigatran N = 40, rivaroxaban N = 110, apixaban N = 42). Plasma clot formation and lysis were recorded continuously on a photometer after addition of an activation mix (tissue factor 2 pmol/l and tissue plasminogen activator 333 ng/ml). We used linear regression and ANCOVA for correlation analysis. Clot formation lag phase was prolonged in the presence of anticoagulants in a concentration-dependent manner for DOACs (dabigatran Spearman r = 0.74; rivaroxaban r = 0.78; apixaban r = 0.72, all p < 0.0001), INR dependent for phenprocoumon (r = 0.59, p < 0.0001), anti-Xa level dependent in LMWH samples (r = 0.90, p < 0.0001). Maximum rate of clot formation and peak clot turbidity were reduced in the presence of anticoagulants, but correlated only moderately with the comparator measures of anticoagulation. The clot lysis time was inversely correlated with DOAC concentrations in the presence of recombinant thrombomodulin. A direct ex vivo comparison between the effects of different classes of anticoagulants is possible with turbidimetric measurement of plasma clot formation and lysis. Anticoagulation inhibited clot formation in a plasma concentration manner for DOACs, INR dependent for phenprocoumon, and anti-Xa dependent for LMWH. Susceptibility to fibrinolysis increased with increasing DOAC concentrations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the introduction of novel direct oral anticoagulants (DOACs) into clinical practice for stroke prevention in atrial fibrillation (AF), the measurement of the intensity of anticoagulation has been challenging. A precise determination of anticoagulation intensity is vital in the clinical management of vitamin K antagonists (VKA), which dominated the landscape of anticoagulation for the past decades, to find the ideal therapeutic window between efficacious prevention of stroke and systemic embolism and safety from bleeding complications [1]. The specific assays that exist to determine the drug concentrations while on treatment with DOACs in patient plasma, including chromogenic anti-Xa assays and diluted thrombin time assays [2,3,4,5], neither indicate the effect of anticoagulation on hemostatic capacity nor are they transferable between different classes of anticoagulants. Global clotting assays, on the other hand, are not linearly dependent and have variable sensitivities to different classes of anticoagulants [6].
Turbidimetric assessment of clot formation and lysis with a high temporal resolution is a simple method to determine clotting and lysis time parameters as well as surrogates for structural clot parameters in an ex vivo, static, tissue-factor-dependent system [7]. The kinetics of clot formation and lysis in samples from “real-world” patients on anticoagulation may give better insights into hemostatic capacity than global clotting assays and specific assays for drug concentration measurements. Ex vivo susceptibility to fibrinolysis after addition of recombinant tissue-type plasminogen activator has further been suggested as a potential biomarker for thromboembolic [8, 9] and bleeding complications [10].
The aim of this study was to test ex vivo plasma clot properties including fibrinolysis potential as a measure of the effect of anticoagulation in plasma samples from real-world patients treated with anticoagulants.
Patients and methods
Patients
We obtained clinical plasma surplus samples from 250 individual patients with a diagnosis of non-valvular AF on treatment with anticoagulation drugs or no treatment, serving as controls. All patients provided written documentation of informed consent. Patients visited the outpatient clinic of the Clinical Division of Hematology and Hemostaseology of the Medical University of Vienna from 2013 to 2016 for routine consultation and assessment of their anticoagulant treatment. Patients were on treatment with dabigatran, rivaroxaban, apixaban, phenprocoumon, or on temporary treatment with low molecular weight heparin (LMWH). The study was approved by the local ethics committee (EC 1711/2014) and is conducted in accordance with the principles set forth by the Declaration of Helsinki. Blood was obtained by atraumatic venous puncture without stasis of the cubital vein using a 21-gauge butterfly needle into a vacuum tube containing trisodium citrate 3.8% (Vacuette® Greiner Bio-One, Kremsmünster, Austria) as part of routine anticoagulation follow-up. We sampled blood in expected peak and expected trough levels in relation to last intake of the anticoagulation drug to obtain a wide variability of anticoagulation intensities. Within 1 h after sampling, citrate blood vials were centrifuged at 2500 g for 15 min at 18 °C (Hettich Rotanta 460Robotic, Tuttlingen, Germany) and the supernatant platelet poor plasma (PPP) was stored in aliquots at − 80 °C until assessment. Patients’ medical histories were recorded as part of the routine risk assessments of stroke and bleeding.
Materials and assays
The prothrombin time (PT), PT-based international normalized ratio (INR), activated partial thromboplastin time (aPTT), fibrinogen, thrombin time, and anti-Xa levels (Hyphen–Biomed, Neuville–sur–Oise, France) were measured in the routine coagulation laboratory of the Department of Medical and Chemical Laboratory Diagnostics of the Medical University of Vienna using an accredited process with fresh plasma immediately after sampling. For all other measurements, the PPP samples were thawed in a water bath at 38 °C for 10 min.
LC–MS/MS
DOAC quantification by LC–MS/MS was facilitated by utilizing a previously established two-dimensional chromatography setup (Agilent Technologies, Waldbronn, Germany) hyphenated to an API 4000 Qtrap instrument (ABSciex, Framingham, MA, USA) operated in the electrospray ionization (ESI) mode [11]. Based on this instrumentation, a research-use-only assay was used as previously published [2]. A calibrator set (nine levels, one blank, Alsachim, Illkirch, France) covering the measurement range of 1.0 (lower limit of quantification [LOQ]) to 600.0 ng/ml (upper LOQ) was used for each DOAC; a linear calibration function with a 1/x weighting factor was employed. Samples with concentrations exceeding the upper LOQ were diluted with blank materials; samples with concentrations below the lower LOQ were not reported. The inter-batch (n = 10) coefficient of variation of the assay was found to be lower than 4.7% for all quality control materials (4, 40, 400 ng/ml); the accuracy of these measurements was better than ± 4.9%. In patient samples, inter-batch coefficients of variation better than 7.6% were found.
Thrombin generation
Thrombin generation was measured using a commercially available assay (Technothrombin TGA, Technoclone, Vienna, Austria), as published previously [12]. Thrombin generation in plasma was initiated with the RC high reagent (high concentration of phospholipid micelles containing 71.6 pM tissue factor in a Tris–Hepes–NaCl buffer, Technoclone).
Plasma clot properties assay
To measure plasma clot properties, we used the static turbidimetric assay, consisting of serial measurements of the optical density (OD) over time in duplicates, described previously [13, 14]. Activation of coagulation in plasma samples is initiated by addition of an activation mix, containing tissue factor (Innovin, Siemens, Marburg, Germany, with final concentration 2 pM), phospholipids (Rossix, Mölndal, Sweden, final 4 µM), and calcium chloride (final 20 nM) inside of transparent 96-well microtiter plates (nunc, Thermofisher Scientific, Bremen, Germany), and the increase in OD during clot formation is closely monitored and recorded in 20 s intervals over 3 h at 405 nm on a Multiskan Spectrum microplate reader (Thermofisher). To measure fibrinolysis potential, we added recombinant tissue plasminogen activator (rt-PA, Actilyse, Boehringer-Ingelheim, 333 ng/ml) simultaneously to the activation mix and measured the decrease in OD during clot lysis.
The parameters of plasma clot properties are automatically derived from the turbidimetric curve (SkanIt Software version 2.4.4, Thermofisher). The lag phase is defined as the time until clot OD surpasses 110% baseline OD and reflects the time required for clot formation [15]. The maximum rate of clot formation is the maximum positive change in clot OD and reflects the maximum rate of fibrin fiber assembly read by fitting a line through 5–10 points of the slope. The peak OD is the maximum achieved OD minus the baseline OD, which reflects the number of protofibrils per fiber and indirectly the structural density of the clot. The clot lysis time is the difference in time from 50% peak OD during clot formation to 50% peak OD during clot lysis and reflects the assembly kinetics as a marker for susceptibility to fibrinolysis [16]. Repeated measures of in-house controls gave an inter-assay coefficient of variation (CV) of 13% and an intra-assay CV of 8.5%.
Thrombomodulin addition
We repeated the plasma clot property assay and the thrombin generation assay in the presence of 10 nM recombinant human thrombomodulin (rThm, Sekisui Diagnostics, Pfungstadt, Germany) maintaining constant concentrations in a subset of 18 samples with a wide range of DOAC concentrations (40–586 ng/ml).
Statistical analysis
Statistical analysis and graphical representation of data were performed using GraphPad Prism (Version 7.00 for Windows, La Jolla, USA) and SPSS (Version 24, IBM, Armonk, USA). Cohort descriptives are given as median and 25th to 75th percentile. The analysis of plasma clot property data includes correlation analysis using Spearman correlation, linear regression for regression analysis, and analysis of covariance (ANCOVA) for comparison of regression equations.
Results
Patients and samples
We obtained 424 citrate plasma samples from 250 patients with AF, of which 400 were on treatment with anticoagulation and 24 did not receive anticoagulation drugs at the time of sampling serving as reference controls. The median CHA2DS2-Vasc score was 4 (25th to 75th percentile 3–5) and the median HAS-Bled 2 (1–2). The median age was 72 years (65–77 years) and the median BMI 27.4 kg/m2 (24.6–31.0 kg/m2). Further characteristics and parameters of the patients, from whom plasma samples were obtained, are given in Table 1.
Plasma clot properties, global assays of coagulation, and clinical factors
The parameters of plasma clot properties did not correlate with global measures of coagulation in controls, but correlated well in samples on treatment with anticoagulation drugs (Table 2). Especially the parameters of clot formation (lag phase, maximum clot formation rate, and peak clot turbidity) correlated well overall with prothrombin time, activated partial thromboplastin time, thrombin time, and fibrinogen in samples on anticoagulation treatment (Table 2). Comorbidities and further clinical factors were weak determinants of plasma clot properties. Patients with diabetes had higher clot formation peak turbidity, patients with congestive heart failure had lower clot formation peak turbidity, patients with arterial vascular disease had longer clot formation lag phase, and women had higher clot formation rates and higher clot formation peak turbidities than men (Table 3). The CHA2DS2-VASc score and the HAS-BLED scores did not correlate with clot formation or lysis parameters. However, in the subset of control samples the CHA2DS2-VASc score correlated moderately well with clot formation peak turbidity (Spearman coefficient 0.507, p = 0.014).
Plasma clot formation properties and specific measures of anticoagulation
The lag phase of clot formation was prolonged in samples on treatment with anticoagulation compared to control samples (Fig. 1a). The increase in clot formation lag phase was positively correlated with the INR in samples from patient on VKA (Fig. 1b), with the LC–MS-/MS-quantified drug concentration in samples from patients on DOACs (Fig. 1c), and with anti-Xa levels in samples from patients on LMWH (Fig. 1d). The slopes of the linear regression equations for DOAC samples were not statistically significantly different (p = 0.274), but the y-intercepts were significantly different (p < 0.0001). Therefore, at corresponding drug concentrations, the effect on lag phase prolongation was strongest in dabigatran samples, followed by rivaroxaban samples and apixaban samples (Fig. 1c).
a Plasma samples from patients on treatment with anticoagulants had prolonged clot formation lag phases compared to controls. The lag phase of clot formation correlated moderately with INR values in VKA patients (b), strongly with drug concentrations measured with LC–MS/MS in samples from patients on DOAC treatment (c), and strongly with anti-Xa levels in samples from patients on treatment with LMWH (d). Only statistically significant correlations are shown with a linear regression line
The maximum rate of clot formation was decreased in samples on treatment with anticoagulants compared to control samples (Fig. 2a). The decrease in the maximum rate of clot formation was negatively correlated with the INR in samples from patient on VKA (Fig. 2b), with the drug concentration in samples from patients on DOACs (Fig. 2c), and with anti-Xa levels in samples from patients on LMWH (Fig. 2d). The slopes of the linear regression equations for DOAC samples were not statistically significantly different (p = 0.402), but the y-intercepts were significantly different (p = 0.0017). Therefore, at corresponding drug concentrations, the maximum rate of clot formation was lowest in dabigatran samples, slightly greater in rivaroxaban samples and highest in apixaban samples (Fig. 2c). The maximum rate of clot formation is further correlated to the parameters of thrombin generation indicating a relationship with the kinetics of thrombin generation (Table 4).
a Plasma samples from patients on treatment with anticoagulants had decreased maximum rate of clot formation compared to controls. The maximum rate of clot formation correlated moderately with INR values in VKA patients (b), moderately with LC–MS/MS measured drug concentrations in samples from patients on rivaroxaban or dabigatran, but less well in samples on apixaban (c), and strongly with anti-Xa levels in samples from patients on treatment with LMWH (d). Only statistically significant correlations are shown with a linear regression line
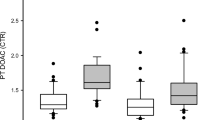
The peak plasma clot turbidity was reduced in samples on treatment with anticoagulants compared to control samples (Fig. 3a). The decrease in the peak plasma clot turbidity was negatively correlated with the INR in samples from patient on VKA (Fig. 3b), with the drug concentration in samples from patients on DOACs (Fig. 3c), and with anti-Xa levels in samples from patients on LMWH (Fig. 3d). The slopes of the linear regression equations for DOAC samples were not statistically significantly different (p = 0.112), but the y-intercepts were significantly different (p = 0.0029). Therefore, at corresponding drug concentrations, peak plasma clot turbidity was lowest in dabigatran samples, greater in rivaroxaban samples, and greatest in apixaban samples (Fig. 3c).
a Plasma samples from patients on treatment with anticoagulants had decreased peak turbidity compared to controls. The peak turbidity of clot formation correlated weakly with INR values in VKA patients (b) and moderately with LC–MS/MS measured drug concentrations in samples from patients on rivaroxaban treatment but only weakly in patients on dabigatran and not in patients on apixaban treatment (c), and moderately with anti-Xa levels in samples from patients on treatment with LMWH (d). Only statistically significant correlations are shown with a linear regression line
Plasma clot lysis time and specific measures of anticoagulation
There was no difference between clot lysis times in samples from patients on anticoagulants compared to controls (Fig. 4a). Clot lysis time also did not correlate significantly with INR in VKA samples or LC–MS-/MS-quantified DOAC concentrations (Fig. 4b–c), but there was a modest trend for a positive correlation between increasing intensity of anticoagulation and increased clot lysis time. The slopes of the linear regression equations for DOAC samples were not significantly different (p = 0.703), but the y-intercepts were significantly different between dabigatran and the Xa inhibitors (p = 0.012). Therefore, at corresponding drug concentrations clot lysis time was longest in dabigatran samples, and shorter in samples on rivaroxaban or apixaban (Fig. 4c). Further, the correlation was strong and significantly positive between clot lysis time and anti-Xa levels in samples from patients on LMWH (Fig. 4d). Plasma clots formed under influence of LMWH with higher anti-Xa levels took longer to lyse after ex vivo addition of rt-PA than in samples with lower anti-Xa levels.
a Plasma samples from patients on treatment with dabigatran and LMWH had increased clot lysis time compared to controls, samples from rivaroxaban and apixaban patients had decreased clot lysis time compared to controls, and samples from VKA patients had indifferent clot lysis time compared to controls. The clot lysis time did not correlate with INR values in VKA patients (b) nor with LC–MS/MS measured drug concentrations in samples from patients on DOAC treatment (c), but strongly and positively with anti-Xa levels in samples form patients on treatment with LMWH (d). Only statistically significant correlations are shown with a linear regression line
Because the clot lysis time was counterintuitively correlated positively with intensity of anticoagulation, and small Spearman r values indicate that the variability in clot lysis time was not accounted for sufficiently by anticoagulation intensity, we explored the relationship between clot lysis time and the parameters of clot formation. In control samples, clot lysis time correlated positively with clot peak turbidity (Spearman r = 0.439, p = 0.032), indicating that the time it takes to lyse is related to the density of the plasma clot (Table 5). The more densely the fibers are assembled, the slower the clot lysis. In samples on anticoagulation, this was not confirmed. Instead parameters that surrogate the rate of clot formation including clot formation lag phase and clot formation maximum rate were correlated to clot lysis time, most strikingly in samples on LMWH (Table 5). This indicates that the slower a clot is formed, the slower the clot is lysed by rt-PA added ex vivo. A comparison with thrombin generation, however, showed that clot lysis time is only weakly correlated with parameters of thrombin generation (Table 4).
Thrombomodulin experiment
To clarify the very modest influence of anticoagulants on clot lysis time, we added rThm. In the presence of rTHM, clot lysis time was prolonged in anticoagulated and control samples (Fig. 5a). The increase in clot lysis time was inversely correlated to DOAC concentration (Fig. 5b), INR level in VKA samples (supplemental Fig. 1A), and anti-Xa levels in LMWH samples (supplemental Fig. 1B). The thrombin generation was reduced in samples with higher concentrations of anticoagulants and addition of rTHM decreased the thrombin generation in comparison to samples without rTHM (Fig. 5c). Clot lysis time and DOAC plasma concentration were inversely correlated only in the presence of rTHM (Fig. 5d).
a Addition of recombinant thrombomodulin (rTHM) prolongs the clot lysis time. b The prolongation of the clot lysis time decreases with increasing DOAC concentration. c Thrombin generation is reduced in the presence of thrombomodulin. d Clot lysis time decreases with increasing DOAC concentration. Only statistically significant correlations are shown with a linear regression line
Discussion
The turbidimetric assay for measuring plasma clot formation and lysis is a useful research method for assessing the effect of anticoagulation. In our investigation, the parameters lag phase, maximum clot formation rate and peak turbidity were correlated across a wide range of DOAC concentration in DOAC samples, INR levels in VKA samples, and anti-Xa levels in LMWH samples. This allows an indirect comparison between the intensity of anticoagulation between different anticoagulant classes. The clot lysis time was only dependent on anticoagulation intensity in the presence of thrombomodulin because anticoagulants reduced the generation of thrombin, which is required for TAFI activation and fibrinolysis inhibition.
The plasma clot formation and lysis assay have previously been used to show the effect of anticoagulants on fibrin clot structure and susceptibility to fibrinolysis [7, 17, 18]. The main objective and novelty of this investigation was to establish comparativeness between different anticoagulants using the plasma clot property assay in patient samples on treatment with anticoagulation. We found that the parameters of clot formation, particularly the lag phase, were strongly correlated with the specific tests for anticoagulation, including plasma concentration of DOACs, INR values in VKA samples, and anti-Xa levels in LMWH samples. Using the regression equations, a cautious comparison between different classes of anticoagulants may be possible. For example, an INR value of 3 in VKA samples corresponded to a lag phase of 591 s, which would correspond to a rivaroxaban concentration of 77.5 ng/ml. The maximum rate of clot formation may be a good parameter to measure the kinetics of the propagation phase of thrombin. We found a strong correlation between PCP maximum rate and parameters of thrombin generation (Table 4). Dabigatran had lower maximum rates of clot formation compared to rivaroxaban and apixaban at corresponding concentrations. There is no direct clinical comparison between DOACs, neither for stroke prevention in AF nor for the treatment of venous thromboembolism. However, it may be clinically relevant to note that corresponding concentrations of different DOACs may result in different impact on hemostasis capacity. Interestingly, in clinical randomized trials dabigatran was the most efficacious in the prevention of strokes, apixaban was safest concerning major bleeding endpoints, and rivaroxaban was somewhat in the middle as a trade-off for the net-clinical benefit [19].
The peak OD of clot formation was only modestly correlated to intensity of anticoagulation in our investigation of real-world samples. Microscopic images previously showed reduced structural clot density under increasing intensities of anticoagulants [17, 18, 20].The variability in peak OD in our samples may be attributed to other determinants, which are difficult to control for in real-world samples compared to studies on normal plasma spiked with anticoagulants [18]. We found that some clinical factors such as sex, diabetes, and heart disease may be able to influence clot properties, although the magnitude of this influence will have to be validated.
There are caveats to be considered when measuring anticoagulation for clinical purposes depending on anticoagulant class [6]. Global assays of anticoagulation such as aPTT and PT are insensitive to low DOAC concentrations [2, 21]; PT is insensitive to dabigatran and poorly sensitive to apixaban, while aPTT is insensitive to rivaroxaban and apixaban [6]. Specific assays such as chromogenic anti-Xa and diluted thrombin time calibrated for Xa-inhibitors and IIa-inhibitors, respectively, are specific but not widely available [22, 23]. Plasma clot properties, although currently not a standardized research tool, may therefore be an adequate measure for comparing the effects on clot formation and fibrinolysis under different anticoagulants.
The other major finding of our investigation was the dependency on thrombomodulin to show a relationship between anticoagulation intensity and fibrinolysis potential. We found an inverse linear relationship between DOAC concentration, INR levels, and anti-Xa, respective to anticoagulant agent, with clot lysis time in real-world plasma samples after addition of rt-PA to initiate fibrinolysis in the presence of rThm (10 nM). In our primary analysis without rThm, there was no correlation between clot lysis time and anticoagulation intensity (Fig. 4b–d), in contrast to previously published results [18]. In the presence of rTHM, we observed a reduction in the thrombin generation (Fig. 5c). Thrombin forms a complex with rThm with great affinity for thrombin-activatable fibrinolysis inhibitor (TAFI) [24], which inhibits fibrinolysis and thus may be responsible for the prolongation of the clot lysis time in our experiment (Fig. 5d), as well as a previous report [25]. The amount of generated thrombin was also dependent on the anticoagulation intensity (Fig. 5c). Therefore, in samples with higher concentrations of an anticoagulant the amount of available thrombin for the rTHM-Thrombin complex was reduced, causing less TAFI activation and less inhibition of fibrinolysis, which we measured as less prolonged clot lysis time. We concluded that anticoagulation intensity and clot lysis time were inversely related (Fig. 5d) and dependent on TAFI. We found that the relationship was the same for dabigatran, rivaroxaban, and apixaban (Fig. 5b), although in a previous investigation, only dabigatran samples had increased the susceptibility to fibrinolysis [26]. The reason may be that the samples in our investigation include some with very high DOAC concentrations (> 500 ng/ml), which were not included in the prior study. An increased susceptibility for fibrinolysis plus the inhibition of clot formation under the influence of anticoagulation may be a two merging drivers for the risk of bleeding in patients on anticoagulation. The use of antifibrinolytic treatment to reduce the risk of bleeding in anticoagulated patients has been postulated previously [27]. Patients with higher susceptibility to fibrinolysis have to be identified with standardized methods for this new approach to reduce bleeding complications while under anticoagulation treatment and improve the net-clinical benefit of anticoagulation treatment in patients with high risk of bleeding. An important issue that demands standardization in the measurement of plasma clot properties is the choice of time points for the measurement of the clot lysis time. For the current investigation, we measured clot lysis time as the difference in time from 50% Δpeak OD during clot formation to 50% Δpeak OD during clot lysis. In measurements that include the entire lag phase and clot formation curve, the clot lysis time is too dependent on the lag phase, which of course is predominantly determined by anticoagulation intensity, which can lead to a false correlation between clot lysis time and anticoagulation intensity. Apart from bleeding endpoints, plasma clot properties may also be associated with thromboembolic endpoints and may offer new possibilities for biomarker guided risk evaluation in other populations [8, 10, 28].
The current investigation is not without limitations. The plasma clot property assay may be more sensitive to thrombin inhibitors because their mode of action is further downstream on the coagulation cascade with a stronger impact on fibrinogen cleavage. However, we found that the regression lines through the data points of different DOACs were almost parallel and we found no exponential relationship between anticoagulation intensity and plasma clot property parameters. Very high concentrations of DOACs (above the limit of quantification of LS-MS/MS [> 600 ng/ml]) yielded poor clotting results and poor thrombin generation measurements, because the tissue factor concentration was too low. The choice of tissue factor concentration was a trade-off to achieve clotting over a wide range of anticoagulation intensities and anticoagulant classes, but with the smallest possible impact of tissue factor and the biggest possible impact of the anticoagulants. We were, however, able to show that such high concentrations of DOACs exist in real-world practice.
Conclusion
In this investigation, we showed a linear relationship between plasma clot properties and specific measures for anticoagulation treatment, respective to the class of anticoagulant. We found that this common measure of tissue-factor-initiated plasma clot formation and lysis might be a method to compare anticoagulants ex vivo. Plasma clot susceptibility to fibrinolysis in real-world samples increased with increasing intensity of anticoagulation across all tested DOACs, VKA, and LMWH, but only in the presence of rThm, suggesting a dependency on TAFI.
References
Hylek EM, Go AS, Chang Y, et al. Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N Engl J Med. 2003;349:1019–26.
Königsbrügge O, Quehenberger P, Belik S, et al. Anti-coagulation assessment with prothrombin time and anti-Xa assays in real-world patients on treatment with rivaroxaban. Ann Hematol. 2015;94:1463–71.
Douxfils J, Dogné J-M, Mullier F, et al. Comparison of calibrated dilute thrombin time and aPTT tests with LC-MS/MS for the therapeutic monitoring of patients treated with dabigatran etexilate. Thromb Haemost. 2013;110:543–9.
Hillarp A, Gustafsson KM, Faxälv L, et al. Effects of the oral, direct factor Xa inhibitor apixaban on routine coagulation assays and anti-FXa assays. J Thromb Haemost. 2014;12:1545–53.
Ruff CT, Giugliano RP, Braunwald E, et al. Association between edoxaban dose, concentration, anti-Factor Xa activity, and outcomes: an analysis of data from the randomised, double-blind ENGAGE AF-TIMI 48 trial. Lancet. 2015;385:2288–95.
Douxfils J, Gosselin R. Laboratory assessment of direct oral anticoagulants. Semin Thromb Hemost. 2017;43:277–90.
Bridge KI, Philippou H, Ariëns RAS. Clot properties and cardiovascular disease. Thromb Haemost. 2014;112:1–8.
Lisman T, Hemost ST, Kwaan H, et al. Decreased plasma fibrinolytic potential as a risk for venous and arterial thrombosis. Semin Thromb Hemost. 2017;43:178–84.
Zabczyk M, Plens K, Wojtowicz W, et al. Prothrombotic fibrin clot phenotype is associated with recurrent pulmonary embolism after discontinuation of anticoagulant therapy. Arterioscler Thromb Vasc Biol. 2017;37:365–73.
Zucker M, Seligsohn U, Salomon O, et al. Abnormal plasma clot structure and stability distinguish bleeding risk in patients with severe factor XI deficiency. J Thromb Haemost. 2014;12:1121–30.
Seger C, Tentschert K, Stöggl W, et al. A rapid HPLC-MS/MS method for the simultaneous quantification of cyclosporine A, tacrolimus, sirolimus and everolimus in human blood samples. Nat Protoc. 2009;4:526–34.
Ay C, Haselböck J, Laczkovics C, et al. Thrombin generation in patients with a bleeding tendency of unknown origin. Ann Hematol. 2011;90:1099–104.
Bajzar L, Fredenburgh JC, Nesheim M. The activated protein C-mediated enhancement of tissue-type plasminogen activator-induced fibrinolysis in a cell-free system. J Biol Chem. 1990;265:16948–54.
Gebhart J, Laczkovics C, Posch F, et al. Plasma clot properties in patients with a mild-to-moderate bleeding tendency of unknown cause. Ann Hematol. 2015;94:1301–10.
Wolberg AS, Gabriel DA, Hoffman M. Analyzing fibrin clot structure using a microplate reader. Blood Coagul Fibrinolysis. 2002;13:533–9.
Jones AJ, Meunier AM. A precise and rapid microtitre plate clot lysis assay: methodology, kinetic modeling and measurement of catalytic constants for plasminogen activation during fibrinolysis. Thromb Haemost. 1990;64:455–63.
Blombäck M, He S, Bark N, et al. Effects on fibrin network porosity of anticoagulants with different modes of action and reversal by activated coagulation factor concentrate. Br J Haematol. 2011;152:758–65.
Varin R, Mirshahi S, Mirshahi P, et al. Whole blood clots are more resistant to lysis than plasma clots–greater efficacy of rivaroxaban. Thromb Res. 2013;131:e100–9.
Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet. 2014;383:2288–95.
Zabczyk M, Blombäck M, Majewski J, et al. Assays of fibrin network properties altered by VKAs in atrial fibrillation—importance of using an appropriate coagulation trigger. Thromb Haemost. 2015;113:851–61.
Dale BJ, Ginsberg JS, Johnston M, et al. Comparison of the effects of apixaban and rivaroxaban on prothrombin and activated partial thromboplastin times using various reagents. J Thromb Haemost. 2014;12:1810–5.
Samama MM, Contant G, Spiro TE, et al. Evaluation of the anti-factor Xa chromogenic assay for the measurement of rivaroxaban plasma concentrations using calibrators and controls. Thromb Haemost. 2012;107:379–87.
Hillarp A, Gustafsson KM, Faxälv L, et al. Effects of the oral, direct factor Xa inhibitor apixaban on routine coagulation assays and anti-FXa assays. J Thromb Haemost. 2014;12:1545–53.
Mosnier LO, Bouma BN. Regulation of fibrinolysis by thrombin activatable fibrinolysis inhibitor, an unstable carboxypeptidase b that unites the pathways of coagulation and fibrinolysis. Arterioscler Thromb Vasc Biol. 2006;26:2445–53.
Ammollo CT, Semeraro F, Incampo F, et al. Dabigatran enhances clot susceptibility to fibrinolysis by mechanisms dependent on and independent of thrombin-activatable fibrinolysis inhibitor. J Thromb Haemost. 2010;8:790–8.
Semeraro N, Incampo F. Dabigatran but not rivaroxaban or apixaban treatment decreases fibrinolytic resistance in patients with atrial fibrillation. Thromb Res. 2016;138:22–9.
He S, Johnsson H, Zabczyk M, et al. A fibrinogen concentrate Haemocomplettan® (Riastap®) or a Factor XIII concentrate Fibrogammin® combined with a mini dose of tranexamic acid can reverse the fibrin instability to fibrinolysis induced by thrombin- or FXa-inhibitor. Br J Haematol. 2013;160:806–16.
Meltzer ME, Lisman T, de Groot PG, et al. Venous thrombosis risk associated with plasma hypofibrinolysis is explained by elevated plasma levels of TAFI and PAI-1. Blood. 2010;116:113–21.
Acknowledgements
The authors would like to acknowledge the technical support by Peter Neyer, Hartwig Pfisterer, Silvia Koder, Florian Posch, and Stefanie Hofer.
Funding
Open Access Funding provided by Medical University of Vienna.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
The ethics committee of the Medical University of Vienna has given approval of the study.
Informed consent
Informed consent was obtained from all individual patients included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Königsbrügge, O., Weigel, G., Quehenberger, P. et al. Plasma clot formation and clot lysis to compare effects of different anticoagulation treatments on hemostasis in patients with atrial fibrillation. Clin Exp Med 18, 325–336 (2018). https://doi.org/10.1007/s10238-018-0490-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-018-0490-9