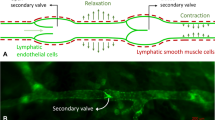
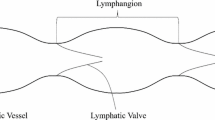
Abstract
A primary purpose of the lymphatic system is to transport fluid from peripheral tissues to the central venous system in order to maintain tissue–fluid balance. Failure to perform this task results in lymphedema marked by swelling of the affected limb as well as geometric remodeling and reduced contractility of the affected lymphatic vessels. The mechanical environment has been implicated in the regulation of lymphatic contractility, but it is unknown how changes in the mechanical environment are related to loss of contractile function and remodeling of the tissue. The purpose of this paper was to introduce a new theoretical framework for acute and long-term adaptations of lymphatic vessels to changes in mechanical loading. This theoretical framework combines a simplified version of a published lumped parameter model for lymphangion function and lymph transport, a published microstructurally motivated constitutive model for the active and passive mechanical behavior of isolated rat thoracic ducts, and novel models for acute mechanically mediated vasoreactive adaptations and long-term volumetric growth to simulate changes in muscle contractility and geometry of a single isolated rat thoracic duct in response to a sustained elevation in afterload. The illustrative examples highlight the potential role of the mechanical environment in the acute maintenance of contractility and long-term geometric remodeling, presumably aimed at meeting fluid flow demands while also maintaining mechanical homeostasis. Results demonstrate that contractility may adapt in response to shear stress to meet fluid flow demands and show that pressure-induced long-term geometric remodeling may attenuate these adaptations and reduce fluid flow. The modeling framework and illustrative simulations help suggest relevant experiments that are necessary to accurately quantify and predict the acute and long-term adaptations of lymphangions to altered mechanical loading.










Similar content being viewed by others
References
Allegra C, Sarcinella R, Bartolo M Jr (2002) Morphologic and functional changes of the microlymphatic network in patients with advancing stages of primary lymphedema. Lymphology 35(3):114–120
Baek S, Gleason RL, Rajagopal KR, Humphrey JD (2007) Theory of small on large: potential utility in computations of fluid–solid interactions in arteries. Comput Methods Appl Mech Eng 196(31–32):3070–3078
Baeyens N, Nicoli S, Coon BG, Ross TD, Van den Dries K, Han J, Lauridsen HM, Mejean CO, Eichmann A, Thomas JL, Humphrey JD, Schwartz MA (2015) Vascular remodeling is governed by a VEGFR3-dependent fluid shear stress set point. Elife 4:e04645
Bertram CD, Macaskill C, Moore JE Jr (2011) Simulation of a chain of collapsible contracting lymphangions with progressive valve closure. J Biomech Eng 133(1):011008. http://doi.org/10.1115/1.4002799
Bertram CD, Macaskill C, Davis MJ, Moore JE Jr (2013a) Development of a model of a multi-lymphangion lymphatic vessel incorporating realistic and measured parameter values. Biomech Model Mechanobiol 13(2):401–416
Bertram CD, Macaskill C, Moore JE Jr (2013b) Incorporating measured valve properties into a numerical model of a lymphatic vessel. Comput Methods Biomech Biomed Eng 17(14):1519–1534
Caulk AW, Nepiyushchikh ZV, Shaw R, Dixon JB, Gleason RL Jr (2015) Quantification of the passive and active biaxial mechanical behaviour and microstructural organization of rat thoracic ducts. J R Soc Interface 12(108):20150280
Davis MJ, Davis AM, Lane MM, Ku CW, Gashev AA (2009) Rate-sensitive contractile responses of lymphatic vessels to circumferential stretch. J Physiol 587(Pt 1):165–182
Davis MJ, Rahbar E, Gashev AA, Zawieja DC, Moore JE Jr (2011) Determinants of valve gating in collecting lymphatic vessels from rat mesentery. Am J Physiol Heart Circ Physiol 301(1):H48–60
Davis MJ, Scallan JP, Wolpers JH, Muthuchamy M, Gashev AA, Zawieja DC (2012) Intrinsic increase in lymphangion muscle contractility in response to elevated afterload. Am J Physiol Heart Circ Physiol 303(7):H795–808
Dixon JB (2010) Lymphatic lipid transport: Sewer or subway? Trends Endocrinol Metab 21(8):480–487
Dixon JB, Greiner ST, Gashev AA, Cote GL, Moore JE, Zawieja DC (2006) Lymph flow, shear stress, and lymphocyte velocity in rat mesenteric prenodal lymphatics. Microcirculation 13(7):597–610
Dixon JB, Zawieja DC, Gashev AA, Cote GL (2005) Measuring microlymphatic flow using fast video microscopy. J Biomed Opt 10(6):064016
Dongaonkar RM, Nguyen TL, Quick CM, Hardy J, Laine GA, Wilson E, Stewart RH (2013) Adaptation of mesenteric lymphatic vessels to prolonged changes in transmural pressure. Am J Physiol Heart Circ Physiol 305(2):H203–210
Fung YC, Liu SQ (1989) Change of residual strains in arteries due to hypertrophy caused by aortic constriction. Circ Res 65(5):1340–1349
Gashev AA, Davis MJ, Gasheva OY, Nepiushchikh ZV, Wang W, Dougherty P, Kelly KA, Cai S, Von Der Weid PY, Muthuchamy M, Meininger CJ, Zawieja DC (2009) Methods for lymphatic vessel culture and gene transfection. Microcirculation 16(7):615–628
Gashev AA, Davis MJ, Zawieja DC (2002) Inhibition of the active lymph pump by flow in rat mesenteric lymphatics and thoracic duct. J Physiol 540(Pt 3):1023–1037
Gasheva OY, Zawieja DC, Gashev AA (2006) Contraction-initiated NO-dependent lymphatic relaxation: a self-regulatory mechanism in rat thoracic duct. J Physiol 575(Pt 3):821–832
Gleason RL, Humphrey JD (2005) A 2D constrained mixture model for arterial adaptations to large changes in flow, pressure and axial stretch. Math Med Biol 22(4):347–369
Gleason RL, Taber LA, Humphrey JD (2004) A 2-D model of flow-induced alterations in the geometry, structure, and properties of carotid arteries. J Biomech Eng 126(3):371–381
Holzapfel G, Gasser T, Ogden R (2000) A new constitutive framework for arterial wall mechanics and a comparative study of material models. J Elast Phys Sci Solids 61(1–3):1–48
Humphrey JD (2008) Mechanisms of arterial remodeling in hypertension: coupled roles of wall shear and intramural stress. Hypertension 52(2):195–200
Humphrey JD, Rajagopal KR (2003) A constrained mixture model for arterial adaptations to a sustained step change in blood flow. Biomech Model Mechanobiol 2(2):109–126
Koller A, Mizuno R, Kaley G (1999) Flow reduces the amplitude and increases the frequency of lymphatic vasomotion: role of endothelial prostanoids. Am J Physiol 277(6 Pt 2):R1683–1689
Kornuta JA, Dixon JB (2014) Ex vivo lymphatic perfusion system for independently controlling pressure gradient and transmural pressure in isolated vessels. Ann Biomed Eng 42(8):1691–1704
Kornuta JA, Nepiyushchikh Z, Gasheva OY, Mukherjee A, Zawieja DC, Dixon JB (2015) Effects of dynamic shear and transmural pressure on wall shear stress sensitivity in collecting lymphatic vessels. Am J Physiol Regul Integr Comp Physiol 00342:02014
Langille BL (1993) Remodeling of developing and mature arteries: endothelium, smooth muscle, and matrix. J Cardiovasc Pharmacol 21(Suppl 1):S11–17
Li B, Silver I, Szalai JP, Johnston MG (1998) Pressure-volume relationships in sheep mesenteric lymphatic vessels in situ: response to hypovolemia. Microvasc Res 56(2):127–138
Liu SQ, Fung YC (1989) Relationship between hypertension, hypertrophy, and opening angle of zero-stress state of arteries following aortic constriction. J Biomech Eng 111(4):325–335
Matsumoto T, Hayashi K (1994) Mechanical and dimensional adaptation of rat aorta to hypertension. J Biomech Eng 116(3):278–283
McHale NG, Roddie IC (1976) The effect of transmural pressure on pumping activity in isolated bovine lymphatic vessels. J Physiol 261(2):255–269
Mihara M, Hara H, Hayashi Y, Narushima M, Yamamoto T, Todokoro T, Iida T, Sawamoto N, Araki J, Kikuchi K, Murai N, Okitsu T, Kisu I, Koshima I (2012) Pathological steps of cancer-related lymphedema: histological changes in the collecting lymphatic vessels after lymphadenectomy. PLoS One 7(7):e41126
Ogata F, Fujiu K, Koshim I, Nagai R, Manabe I (2014) Phenotypic modulation of smooth muscle cells in lymphedema. Br J Dermatol 172(5):1286–1293
Olszewski WL (2008) Contractility patterns of human leg lymphatics in various stages of obstructive lymphedema. Ann N Y Acad Sci 1131:110–118
Quick CM, Venugopal AM, Gashev AA, Zawieja DC, Stewart RH (2007) Intrinsic pump-conduit behavior of lymphangions. Am J Physiol Regul Integr Comp Physiol 292(4):R1510–1518
Rachev A, Hayashi K (1999) Theoretical study of the effects of vascular smooth muscle contraction on strain and stress distributions in arteries. Ann Biomed Eng 27(4):459–468
Rahbar E, Akl T, Cote GL, Moore JE Jr, Zawieja DC (2014) Lymph transport in rat mesenteric lymphatics experiencing edemagenic stress. Microcirculation 21(5):359–367
Rahbar E, Moore JE Jr (2011) A model of a radially expanding and contracting lymphangion. J Biomech 44(6):1001–1007
Reddy NP, Krouskop TA, Newell PH Jr (1977) A computer model of the lymphatic system. Comput Biol Med 7(3):181–197
Rodriguez EK, Hoger A, McCulloch AD (1994) Stress-dependent finite growth in soft elastic tissues. J Biomech 27(4):455–467
Scallan JP, Wolpers JH, Davis MJ (2013) Constriction of isolated collecting lymphatic vessels in response to acute increases in downstream pressure. J Physiol 591(Pt 2):443–459
Scallan JP, Wolpers JH, Muthuchamy M, Zawieja DC, Gashev AA, Davis MJ (2012) Independent and interactive effects of preload and afterload on the pump function of the isolated lymphangion. Am J Physiol Heart Circ Physiol 303(7):H809–824
Taber LA (1998) A model for aortic growth based on fluid shear and fiber stresses. J Biomech Eng 120(3):348–354
Valentín A, Cardamone L, Baek S, Humphrey JD (2009) Complementary vasoactivity and matrix remodelling in arterial adaptations to altered flow and pressure. J R Soc Interface 6(32):293–306
Wan W, Hansen L, Gleason R Jr (2010) A 3-D constrained mixture model for mechanically mediated vascular growth and remodeling. Biomech Model Mechanobiol 9(4):403–419
Wolinsky H (1970) Response of the rat aortic media to hypertension. Morphological and chemical studies. Circ Res 26(4):507–522
Zawieja DC, Davis KL, Schuster R, Hinds WM, Granger HJ (1993) Distribution, propagation, and coordination of contractile activity in lymphatics. Am J Physiol 264(4 Pt 2):H1283–1291
Zhang RZ, Gashev AA, Zawieja DC, Davis MJ (2007) Length-tension relationships of small arteries, veins, and lymphatics from the rat mesenteric microcirculation. Am J Physiol Heart Circ Physiol 292(4):H1943–1952
Acknowledgments
We gratefully acknowledge that this study was supported by funds awarded from the National Institutes of Health (R01-HL113061).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Caulk, A.W., Dixon, J.B. & Gleason, R.L. A lumped parameter model of mechanically mediated acute and long-term adaptations of contractility and geometry in lymphatics for characterization of lymphedema. Biomech Model Mechanobiol 15, 1601–1618 (2016). https://doi.org/10.1007/s10237-016-0785-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-016-0785-2