Abstract
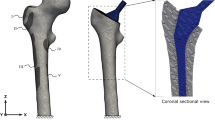
Although research has been addressed at investigating the effect of specific loading regimes on bone response around the implant, a precise quantitative understanding of the local mechanical response close to the implant site is still lacking. This study was aimed at validating micro-CT-based finite element (μFE) models to assess tissue strains after implant placement in a rat tibia. Small implants were inserted at the medio-proximal site of 8 rat tibiae. The limbs were subjected to axial compression loading; strain close to the implant was measured by means of strain gauges. Specimen-specific μFE models were created and analyzed. For each specimen, 4 different models were created corresponding to different representations of the bone–implant interface: bone and implant were assumed fully osseointegrated (A); a low stiffness interface zone was assumed with thickness of 40 μm (B), 80 μm (C), and 160 μm (D). In all cases, measured and computational strains correlated highly (R 2 = 0.95, 0.92, 0.93, and 0.95 in A, B, C, and D, respectively). The averaged calculated strains were 1.69, 1.34, and 1.15 times higher than the measured strains for A, B, and C, respectively, and lower than the experimental strains for D (factor = 0.91). In conclusion, we demonstrated that specimen-specific FE analyses provide accurate estimates of peri-implant bone strains in the rat tibia loading model. Further investigations of the bone-implant interface are needed to quantify implant osseointegration.
Similar content being viewed by others
References
Adams M (2002) Evaluation of three unstructured multigrid methods on 3D finite element problems in solid mechanics. Int J Numer Methods Eng 55: 519–534
Arbenz P, van Lenthe GH, Mennel U, Müller R, Sala M (2008) A scalable multi-level preconditioner for matrix-free μ-finite element analysis of human bone structures. Int J Numer Methods Eng 73: 927–947
Boyd SK, Muller R, Zernicke RF (2002) Mechanical and architectural bone adaptation in early stage experimental osteoarthritis. J Bone Miner Res 17(4): 687–694
Branemark PI, Hansson BO, Adell R, Breine U, Lindstrom J, Hallen O, Ohman A (1977) Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand J Plast Reconstr Surg Suppl 16: 1–132
Brunski JB (1999) In vivo bone response to biomechanical loading at the bone/dental-implant interface. Adv Dent Res 13: 99–119
Carter DR, Giori NJ (1991) Effect of mechanical stress on tissue differentiation in the bony implant bed. In: Davies JE (eds) The bone-biomaterial interface. University of Toronto Press, Toronto, pp 367–376
Chang MC, Ko CC, Liu CC, Douglas WH, DeLong R, Seong WJ, Hodges J, An KN (2003) Elasticity of alveolar bone near dental implant-bone interfaces after one month’s healing. J Biomech 36(8): 1209–1214
Davies JE (2003) Understanding peri-implant endosseous healing. J Dent Educ 67(8): 932–949
De Smet E, Jaecques SV, Jansen JJ, Walboomers F, Vander SJ, Naert IE (2007) Effect of constant strain rate, composed of varying amplitude and frequency, of early loading on peri-implant bone (re)modelling. J Clin Periodontol 34(7): 618–624
De Smet E, Jaecques SV, Jansen JJ, Walboomers F, Vander SJ, Naert IE (2008) Effect of strain at low-frequency loading on peri-implant bone (re)modelling: a guinea-pig experimental study. Clin Oral Implants Res 19(8): 733–739
De Smet E, Jaecques SVN, Wevers M, Jansen JA, Jacobs R, Sloten JV, Naert IE (2006) Effect of controlled early implant loading on bone healing and bone mass in guinea pigs, as assessed by micro-CT and histology. Eur J Oral Sci 114: 232–242
Duyck J, Corpas L, Vermeiren S, Ogawa T, Quirynen M, Vandamme K, Jacobs R, Naert I (2010) Histological, histomorphometrical, and radiological evaluation of an experimental implant design with a high insertion torque. Clin Oral Implants Res 21(8): 877–884
Friberg B, Sennerby L, Grondahl K, Bergstrom C, Back T, Lekholm U (1999) On cutting torque measurements during implant placement: a 3-year clinical prospective study. Clin Implant Dent Relat Res 1(2): 75–83
Gerhard FA, Lambers FM, Kuhn G, Müller R (2008) Rigid registration allows quantification of bone formation and resorption in a longitudinal in vivo mouse study of bone adaptation. In: Annual meeting Swiss society for biomedical engineering Muttenz (Switzerland) 4–5 September, p 14
Huja SS, Katona TR, Burr DB, Garetto LP, Roberts WE (1999) Microdamage adjacent to endosseous implants. Bone 25(2): 217–222
Ko CC, Douglas WH, DeLong R, Rohrer MD, Swift JQ, Hodges JS, An KN, Ritman EL (2003) Effects of implant healing time on crestal bone loss of a controlled-load dental implant. J Dent Res 82(8): 585–591
Leucht P, Kim JB, Wazen R, Currey JA, Nanci A, Brunski JB, Helms JA (2007) Effect of mechanical stimuli on skeletal regeneration around implants. Bone 40(4): 919–930
Lioubavina-Hack N, Lang NP, Karring T (2006) Significance of primary stability for osseointegration of dental implants. Clin Oral Implants Res 17(3): 244–250
Matsuyama J, Ohnishi I, Sakai R, Suzuki H, Harada A, Bessho M, Matsumoto T, Nakamura K (2006) A new method for measurement of bone deformation by echo tracking. Med Eng Phys 28(6): 588–595
Mavrogenis AF, Dimitriou R, Parvizi J, Babis GC (2009) Biology of implant osseointegration. J Musculoskelet Neuronal Interact 9(2): 61–71
Natali AN, Carniel EL, Pavan PG (2009) Dental implants press fit phenomena: biomechanical analysis considering bone inelastic response. Dent Mater 25(5): 573–581
Ogawa T, Possemiers T, Zhang X, Naert I, Chaudhari A, Sasaki K, Duyck J (2011) Influence of whole-body vibration time on peri-implant bone healing: a histomorphometrical animal study. J Clin Periodontol 38(2): 180–185
Ogawa T, Zhang X, Naert I, Vermaelen P, Deroose CM, Sasaki K, Duyck J (2011) The effect of whole-body vibration on peri-implant bone healing in rats. Clin Oral Implants Res 22(3): 302–307
Rubin CT, McLeod KJ (1994). Promotion of bony ingrowth by frequency-specific, low-amplitude mechanical strain. Clin Orthop Relat Res (298):165–174
Shalabi MM, Wolke JG, de Ruijter AJ, Jansen JA (2007) Histological evaluation of oral implants inserted with different surgical techniques into the trabecular bone of goats. Clin Oral Implants Res 18(4): 489–495
Tabassum A, Meijer GJ, Wolke JG, Jansen JA (2009) Influence of the surgical technique and surface roughness on the primary stability of an implant in artificial bone with a density equivalent to maxillary bone: a laboratory study. Clin Oral Implants Res 20(4): 327–332
Tabassum A, Walboomers XF, Wolke JG, Meijer GJ, Jansen JA (2010) Bone particles and the undersized surgical technique. J Dent Res 89(6): 581–586
Torcasio A, van Lenthe GH, Van OH (2008) The importance of loading frequency, rate and vibration for enhancing bone adaptation and implant osseointegration. Eur Cell Mater 16: 56–68
Torcasio A, Zhang X, Duyck J, van Lenthe GH (2011) 3D characterization of bone strains in the rat tibia loading model. Biomech Model Mechanobiol. doi:10.1007/s10237-011-0320-4
van Lenthe GH, Müller R (2008) CT-based visualization and quantification of boe microstructure in vivo. IBMS BoneKEy 5(11): 410–425
van Lenthe GH, Voide R, Boyd SK, Muller R (2008) Tissue modulus calculated from beam theory is biased by bone size and geometry: implications for the use of three-point bending tests to determine bone tissue modulus. Bone 43(4): 717–723
van Rietbergen B, Weinans H, Huiskes R, Odgaard A (1995) A new method to determine trabecular bone elastic properties and loading using micromechanical finite-element models. J Biomech 28(1): 69–81
Warreth A, Polyzois I, Lee CT, Claffey N (2009) Generation of microdamage around endosseous implants. Clin Oral Implants Res 20(12): 1300–1306
Wirth AJ, Mueller TL, Vereecken W, Flaig C, Arbenz P, Müller R, van Lenthe GH (2010) Mechanical competence of bone-implant systems can accurately be determined by image-based micro-finite element analyses. Arch Appl Mech 80: 513–525
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Torcasio, A., Zhang, X., Van Oosterwyck, H. et al. Use of micro-CT-based finite element analysis to accurately quantify peri-implant bone strains: a validation in rat tibiae. Biomech Model Mechanobiol 11, 743–750 (2012). https://doi.org/10.1007/s10237-011-0347-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-011-0347-6